Follow along: IG @birectifier
Kervegant Chapter IV The Yeasts: [PDF]
Pages 94 – 125
CHAPTER IV THE YEASTS (1)
(1) Guilliermond (A) et Tanner (F. J.) – The Yeasts, New York, 1919.
What we commonly call yeasts are all microorganisms which, placed in a sugar solution, give rise to alcohol and carbon dioxide, that is to say determine alcoholic fermentation.
In the botanical sense of the word, we thus designate unicellular fungi, having oval or round forms, multiplying by budding or fission and producing ascopores [spores] (Guilliermond). Yeasts are from the family Saccharomycetaceae, which forms with that of Endomycetaceae, the group of Protoasca, or Ascomycetes inferior.
[not sure if the last italicized terms are conjugated properly.]
In addition to these well-characterized yeasts, there are others that never give asci. It is difficult to say whether they are true yeasts that have lost their ability to produce spores, or whether they represent forms derived from higher fungi fixed in the yeast state. Therefore, they are classified in a provisional group, referred to as Non-Saccharomycetaceae.
Finally, there are mycelial fungi that can give birth to budding cells in the form of yeasts, which can multiply in turn for several generations by budding. In other fungi, the mycelium disarticulates into rectangular cells (anthrospores), capable of continuing to divide by transverse partitioning like Schizosaccharomyces. These organisms are usually referred to as yeast-shaped fungi.
Yeasts are very common in nature: they are found in the air, soil, on the surface of plants (fruits, leaves, etc.), especially when they contain sugar. In cold and temperate regions they overwinter in the soil, but in the tropics they persist year-round on plants. They are found especially on the surface of cane stems, represented by many species (Saccharomyces, Torula, etc.) and accompanied by molds (Aspergillus, etc.) and bacteria (acetic, butyric, lactic).
Morphology, development and composition.
Yeasts are generally isolated cells, very polymorphic (round, oval, elliptical, lemon, pear, baton, etc.) measuring 1 to 9 mus long and 1 to 5 mus broad. Shape and dimensions vary with yeast age and acidity of the culture medium for example determines cell elongation, while oxygen makes them become oval or globular. Therefore, it is not possible to rely solely on morphological characters to identify species. There is, however, often in a crop a predominant form, sometimes characteristic (apiculate yeasts, torulas, etc.).
Yeast reproduction is usually done by budding, but it can also be done by fission or by sporulation.
In multiplication by budding, a small bud is formed on the surface of the yeast cell, which grows little by little and soon takes on the size of the mother cell. The nucleus divides by amitosis. Young cells detach themselves before they have reached their full development, or remain adherent for some time; then each cell starts to proliferate in turn. When cells remain united in bundles or rosaries which may have 15-20 cells, on a top fermenting yeast, which rises to the surface of musts, raised by carbon dioxide, and forms a sort of hat. When, on the contrary, cells are isolated or united in pairs, they constitute a bottom fermenting yeast, which remains at the bottom of the fermentation vats. [ale versus lager]
Yeasts of the genus Schizosaccharomyces are characterized by their multiplication by fission: the cell elongates and, having reached a certain size, forms a median partition, diversely directed, which divides it into two daughter cells. These may separate immediately or remain united for some time, constituting excrescences in the form of very elongated skins. Under certain conditions (air deficiency in particular), they have a marked tendency to remain adherent and to form branched chains.
When fermentation is finished and the liquid has become immobile, the yeast cells may continue to develop aerobically: they form on the surface of the liquid a veil, a crown, or a ring on contact line of the surface with the receptacle. The conditions of formation of the veil (speed, temperature limits, etc.), as well as the appearance of it, constitute characters making it possible to differentiate the species. In some cases, the veil appears at the beginning of fermentation (Willia, Mycoderma, etc.); in others, it is not formed at all.
Spores are yeast resistance organs against external agents. It is explained, by Hansen, that they are formed only from young and vigorous cells, placed in unfavorable dietary conditions, in the presence of oxygen and between certain temperature limits, variable with the yeast races. Spores are found in veils, and can be easily obtained by placing young, well-nourished yeast in some solid media. The number of spores per cell, or asci, varies between 1 and 12, and is often characteristic of a race or species of yeast. Their dimensions range from 1.5 to 5 mus. Ascospores are usually spherical or oval, but sometimes have characteristic forms: hemispherical, triangular, limoniform, etc. The mode of germination of the spores is also sometimes characteristic of the species.
In some yeasts, asci results from copulation of two cells of equal size (isogamy) or unequal (heterogamy). In the first case, two similar cells unite with one another by a thin canal; the spores form in the two bulges of the ascus, which looks like a dumbbell. In the second case, a small cell unites by a channel to a large cell; the contents of the first emigrate in the second, where the ascopores are born.
Isogamic conjugation is found in the genera Shizosaccharomyces and Zygosaccharomyces; heterogamic conjugation in the genera Deburyomyces, Nadsonia and some Zygosaccharomyces. In addition, there are intermediate cases between iso and heterogamy. Some yeasts (Schwanniomyces, Torulaspora) present only remnants of sexuality: the cells can make several attempts to join together, without achieving it, and give rise to several filaments, which radiate around them. Finally, in some species (s. Ludwigii for example), when the conjugate can not take place at the time of the formation of asci, a copulation occurs between ascopores (parthenogamy).
Chemical composition of yeasts is very variable according to culture conditions and race studied. For a given yeast, it also changes, at least quantitatively, as the fermentation progresses.
Water content is always high and around 70-75%. Composition of the dry matter varies between the following limits:  The most important carbohydrates are glycogen, a reserve substance that can reach 38% at the end of fermentation, and mannane found in the cell membrane.
The most important carbohydrates are glycogen, a reserve substance that can reach 38% at the end of fermentation, and mannane found in the cell membrane.
The fat content generally reaches 2-5% of the dry matter. It can, however, rise to 20% in old degenerate crops and even, for some breeds, up to 50%. The fats of the yeast are mainly composed of the esters of palmitic, lauric, linolic and arachidic acids. There are also small quantities of lecithins and phytosterols (ergosterol).
Nitrogenous or proteinaceous materials are 90% formed by true proteins (cerevisin, zymocasein) and nucleins. Non-proteinaceous nitrogenous materials include peptones, amino acids (leucine, valine, lysine, etc.), and amides (xanthine, hypoxanthine, guanine) Meisenheimer (1), who studied nitrogenous yeast materials by hydrolysis in the presence of toluene, found among the protein degradation products, all common amino acids, including glucosamine, which presence was not detected until then. According to this author, yeast nitrogen would be distributed as follows:
(1) Woch, Brauerei XXXII, 325, 1915.  As for mineral substances, they consist mainly of phosphoric acid (45 to 60% of ash), potash (30 to 40%) and magnesia (4 to 8%). Lime, soda, silica, iron, sulfur and chlorine are also present in small quantities. The proportion of the various substances above varies, moreover, within wide limits, according to the chemical composition of the medium in which the yeast has developed. This observation applies especially to lime and magnesia, which may be very abundant or in very small quantities.
As for mineral substances, they consist mainly of phosphoric acid (45 to 60% of ash), potash (30 to 40%) and magnesia (4 to 8%). Lime, soda, silica, iron, sulfur and chlorine are also present in small quantities. The proportion of the various substances above varies, moreover, within wide limits, according to the chemical composition of the medium in which the yeast has developed. This observation applies especially to lime and magnesia, which may be very abundant or in very small quantities.
Yeast Nutrition.
Like all living beings, yeast need, for maintenance and development, nitrogen, hydrocarbon and mineral matter. It is important, moreover, to distinguish the action of these substances on cell multiplication on the one hand, and on zymatic function, that is, on fermentation, on the other. There is frequently antagonism between plant function and ferment function.
Mineral matter.
The role played by mineral materials in yeast multiplication was first studied by Mayer (2). This author has found that the most favorable nutritive medium had the following composition, which corresponds, with regard to the equilibrium of various mineral elements, to that of yeast ashes:
(2) Lehrbuch der Gärungschemie. 5° éd. 1902.  Phosphates and potash are of paramount importance in the nutrition of yeast. Sulfur is also essential.
Phosphates and potash are of paramount importance in the nutrition of yeast. Sulfur is also essential.
Lime and magnesia, which can not be replaced by each other, do not seem absolutely necessary, but nevertheless play a role in the internal chemistry of the cell which renders them extremely useful. In fact, it has been found that yeast degenerates rapidly in lime-free environments, Hayduck and Kenneberg have also observed that, placed in a solution of pure sugar, bear yeasts die very quickly, but that if one adds in the middle a small quantity of lime salts, their vitality is much greater. It is likely that the death of the cells is determined by the formation within them of acids, which are neutralized in the presence of lime or other alkalis. [always be buffering!]
Mayer considered iron salts useless. However other authors (Molich, Wehmer) consider that they exert a favorable action on yeast multiplication.
Phosphates and sulfur also have a considerable influence on zymatic function. Elion has been able to observe that monopotassium phosphate and neutral phosphate of ammonia produce an increase in the release of carbonic acid, which varies, for a given time, between 23 and 63% according to the yeasts. Harden and Young have shown that soluble phosphates play an essential role in the action of alcoholic zymase.
Stern has observed that in a nutrient medium completely deprived of sulfur, complete fermentation of the sugar can not be achieved. But if Ca sulphate and Mg sulphate are added, transformation of the sugar is complete.
When they exceed a certain proportion in the culture medium, mineral elements do not intervene any more usefully in yeast nutrition, nor in fermentation. Stern observed that beyond 250 mgr per liter of sugar solution (added with 250 mg of nitrogen in the form of asparagine), there is no longer any increase in the quantity of nitrogen assimilated, yeast weight nor sugar consumed.
Independently of the above elements, recognized as essential for development and zymatic yeast function, there are other mineral salts which, in moderate doses, exert a favorable action. It has thus been recognized that ferment power is increased by small doses of manganese salts (Kayser and Marchand), tin protochloride and bismuth sub-nitrate (Gimel), copper sulphate and sodium cyanide (Hildebrandt and Boyce), etc. … Aluminum salts also have a slight stimulating action on fermentation and cell multiplication (Sikes), while lithium salts are harmful.
Nitrogenous substances.
Proteins themselves (albumins, casein, fibrin), not diffusible through cell membranes, constitute bad nitrogenous foods. According to Pasteur and Mayer, yeasts can not assimilate egg white albumin or blood fibrin. Under certain conditions, however, complex nitrogenous materials could be used. Thus, Boullanger found that milk, seeded with certain yeasts, coagulates little by little and that after a few months the coagulum is liquefied, with formation of ammoniacal salts, tyrosine and leucine: there was thus dissolution and digestion of casein.
On the other hand, albumins and peptones are good yeast foods. Duclaux (1) has shown that nitrogenous substances contained in the yeast water, and which consist mainly of peptones, would even have more favorable action on cell multiplication than ammoniacal salts. Hayduck, experimenting with asparagine and peptone, found that the latter favored yeast multiplication much more than asparagine.
(1) Traité de Microbiologie – t. III. La fermentation alcoolique. Paris, 1905.
More advanced degradation products of albuminoids (amides, amino acids, etc.) are assimilated more easily than albumins. However, assimilability varies with the nature of these substances. Some amino acids, such as leucine, isoleucine, adenine, aspartic acid, are easily absorbed, while others (histidine, choline, thymine, hypoxanthine) are difficult to absorb. Of the amides, allantoin, asparagine and urea are assimilated, but not creatine, creatinine or succinamic acid.
According to Lindner (1), compounds whose hydrocarbon groups are in long open chains (leucine, adenine, lysine) are more easily absorbed than those with a closed chain (histidine, thymine, choline). Yeast race is also of great importance: the most aerobic yeasts use nitrogen compounds that are difficult to easily assimilate.
(1) Chem. Ztg XXXIV. 1144, 1910.
Carbon of amino acids and amides can not be used for yeast nutrition. Ehrlich showed that nitrogen was absorbed, probably in the form of ammonia, and transformed into proteic matter, while the rest of the molecule was released into the medium in the form of higher alcohols. Effront has also reported existence of amidase, which acts on amides, giving rise to ammonia and volatile acids.
Ammoniacal salts are very well absorbed by yeasts, as found by many experimenters (Pasteur, Duclaux, Mayer, etc.)
In the presence of a mixture of ammoniacal nitrogen and organic nitrogen, yeasts preferably first attack the ammoniacal salts. But these, as Ducluaux has shown, have a much more favorable effect on the functioning of zymase than on cell multiplication. Here are the increases in dry yeast obtained with various nitrogenous materials (Bokorny) (2):
(2) Chem. Ztg. XL, 366, 1916.  In contrast to higher plants, nitrates are poorly used by yeast. According to Laurent, they could not be assimilated and, by transforming themselves into nitrites, they would exert a toxic action on yeasts. However, the experiments of Kayser (3) and those of Ferebach and Lanzerberg (4) have shown that if nitrates mediate cell multiplication, they activate the action of zymase and promote fermentation. According to Nicoleau (5), nitric nitrogen shows its accelerating action on yeast ferment from a nitrate concentration of about 5%, with a variable optimum depending on the medium, the initial quantity of seed, the vigor yeast, etc.
In contrast to higher plants, nitrates are poorly used by yeast. According to Laurent, they could not be assimilated and, by transforming themselves into nitrites, they would exert a toxic action on yeasts. However, the experiments of Kayser (3) and those of Ferebach and Lanzerberg (4) have shown that if nitrates mediate cell multiplication, they activate the action of zymase and promote fermentation. According to Nicoleau (5), nitric nitrogen shows its accelerating action on yeast ferment from a nitrate concentration of about 5%, with a variable optimum depending on the medium, the initial quantity of seed, the vigor yeast, etc.
(3) C. R. CXLIV, 574 1907, CLI 816. 1910.
(4) C.R CLI. 726, 1910.
(5) Les nitrates dans la vie de la levure. Paris 1924.
As a final result of studies that have been done on the subject, foods of choice for yeast development and multiplication are complex nitrogenous materials in the form of peptones, while for the exercise of fermentation functions, this nitrogenous matter is deeply degraded and as close as possible to the form of ammonia.
Above a certain proportion, nitrogenous materials have an unfavorable effect on fermentation. According to Pringsheim, the quantity of nitrogen giving best results in terms of alcohol yield varies, depending on yeast races and environmental conditions, between 0.004 and 0.008%.
Hydrocarbon materials or carbohydrates.
Hydrocarbon metabolism of yeasts is different, depending on whether they live in aerobiosis (plant function) or in anaerobiosis (fermentative or zymatic function).
In aerobic life, yeast can use many organic compounds as maintenance foods. According to Laurent’s research (1), it is capable of assimilating the carbon of organic salts (acetates, lactates, citrates, tartrates, malates, succinates), organic acids (citric, tartaric, lactic succinic), ethyl alcohol and polyalcohols (glycerol, mannite), C6 and C12 sugars, and bodies capable of giving sugars, glucosides or dextrins. On the other hand, esters, fatty acids (in the form of acids), amides, glycine, hydroquinone, cellulose, etc. could not feed the yeast.
(1) Ann. Inst. Pasteur TII, 1889.
Bokorny came to similar conclusions. It seems, however, that under certain conditions and for certain species, yeasts may use ethyl alcohol (Trillat, Kayser, Lindner).
Maltose appears to be the sugar most easily assimilated by aerobic yeast; sucrose, glucose, levulose, raffinose are less easily, while lactose and dextrins are used only in special cases. There is no relation between the fermentability of a sugar and its assimilability in aerobiosis: Schizosaccharomyces Pombe, for example, which actively ferments sucrose, glucose and levulose, is unable to assimilate them (Linder and Saito) (2).
(2) Woch. Braurei XXVII, N° 41. 1910.
In an anaerobic or fermentary life, yeast attacks only certain sugars and polysaccharides giving rise to them by hydrolysis.
According to Fischer and Thierfelder (3), only sugars containing 3 carbon atoms (or a multiple of 3) are fermentable. However, there appear to be exceptions to this rule: pentoses (arabinose, xylose), for example, may be attacked by certain yeasts (Lindner). On the other hand, the trioses appear to be transformed into hexoses before any fermentation. Virtually only the following 3 hexoses: d-glucose, d-fructose (levulose) and d-mannose, are directly and easily attackable. Fermentation of d-galactose is only obtained with yeasts acclimated to this sugar.
(3) Ber. Deut. Chem. Ges. XXVII, 2114, 1894.
Yeast shows a marked preference for one or the other of the fermentable hexoses (phenomenon of the elective fermentation). In a mixture of glucose and levulose, for example, it is found that glucose usually disappears more rapidly than levulose at the beginning of fermentation. Subsequently, the phenomenon is reversed, so that finally, there remains in the liquid an excess of glucose. Some breeds, however, are an exception: Sauternes yeasts, described by Dubourg, make levulose disappear more rapidly than glucose from the beginning to the end of fermentation. The elective property also depends on the constitution of the culture medium and temperature. Thus, in the presence of manganese salts, levulose disappears faster than glucose (Kayser).
Disaccharides (sucrose, maltose, lactose, trehalose) and trisaccharides (raffinose) are attacked only if the yeast possesses the necessary diastases for their hydrolysis into fermentable hexoses. Most species ferment sucrose, much maltose, but little attack raffinose and trehalose.
As for the polysaccharides (starch, dextrins, inulin, etc.), they can undergo alcoholic fermentation under the action of certain fungi (Mucors), but they are only attacked quite exceptionally by the yeasts. It should be noted, however, that Schizosaccharomyces Pombe and Schizosaccharomyces mellacei, for example, ferment dextrin and inulin.
Ferment power and yeast activity.
The term “ferment power” designates the ratio of the quantity of sugar consumed to the quantity of yeast produced, that is to say the quantity of sugar which the unit of weight of this yeast is capable of making disappear. This power varies according to yeast race and conditions of the culture medium. Certain breeds, especially those used in the manufacture of industrial alcohol, can thoroughly push the fermentation of must rich in sugar while others stop before having processed all the sugar.
Ferment power represents, as Lindet (1) points out, the sum of the vegetable power and the zymase power, that is to say, the quantities of sugar used by the yeast for its maintenance and its development on the one hand; for the fermentation of alcohol under the action of zymase on the other hand. These two powers are complementary, so that the weight of alcohol formed decreases when the amount of sugar used for the functioning of plant life increases. Lindet has shown that the fermentation is slower if the vegetable function is of more importance than the zymatic function. The lower the amount of yeast at the beginning, the longer the fermentation is, and the lower the yield of alcohol. However, when we exceed a certain limit (1 per 1000 yeast supposedly dry), we obtain a very rapid fermentation, but then the maintenance and breathing of an excessive number of cells determine an exaggerated consumption of sugar in the vegetable phase.
(1) C. R. CLXIV, 58, 1917 : CLXVI, 910, 1918.
The activity of a yeast is the amount of sugar that the unit weight of the yeast makes disappear in the unit of time. This activity also varies with yeast races and with environmental conditions.
Finally, a yeast is said to have low or high attenuation limit, depending on the amount of fermentable sugars remaining in the must at the end of the fermentation is more or less important. If we compare their action in sweet liquids of various compositions, the races of yeast always rank in the same order. This made it possible to group them industrially in yeasts with low attenuation (Saaz type), medium attenuation (Frohberg type) and high attenuation (Logos type, Pombe yeast). [schizosaccharomyces]
The attenuation power of a yeast is not only due to its ability to ferment carbohydrates, but also to its resistance to increasingly unfavorable environmental conditions as and when that the fermentation advances (increase of acidity, depletion of the must, etc.). “In short, the attenuation limit of a must does not correspond to the disappearance of any fermentable material, the stopping (at least apparent) of the fermentation is due to a set of factors that slow down the phenomenon more and more energetically” (Van Laer).
In industrial alcohol distilleries, preference is given to yeasts whose ferment power and activity are high, that is to say which give in a short time a high production of alcohol. It also seeks to achieve the conditions that bring fermentative power and activity to their maximum. This is not always the case in the manufacture of eaux-de-vie, where, in order to obtain aromatic products, it is often advantageous to slow down the fermentation. Sometimes even in the fermented beverages industry, it is advisable to address low attenuation yeasts, leaving in the liquid a certain quantity of unprocessed sugar (making sweet cider, for example).
Diastases.
The living cell’s chemical reactions (hydrolyses, oxidations and reductions, various condensations) are made by means of “biochemical catalysts”, called diastases or enzymes.
These are divided into two major groups: digestion diastases or hydrolases, which hydrolyze food, and respiration or fermentation diastases, desmolases, which carry out the oxidation and reduction phenomenon, and disruption of organic molecules.
In the first group, there are esterases, which cause esterification and saponification, and glucidases, which hydrolyze carbohydrates and nitrogenases, which hydrolyze proteins. The best known of the desmolases is the alcoholic zimase.
The various yeast species are differentiated from one another by the diastases, and more particularly by the glucidases, which they secrete.
Yeast cells contain several proteolytic diastases, best known of which is endotryptase. This, probably consisting of a mixture of several diastases, is closer to trypsin than pepsin because it pushes the degradation of albuminoid materials to the amino acid stage (Geret and Hahn). However, it is distinguished from trypsin in that it is favored by a clearly acidic reaction (optimum: 0.2% in HCl). It is hindered by alkalis, sugars, alcohol 10%. It begins to act at 5-6°, has the temperature optimum of 45° and is destroyed by heating for one hour at 60°. Endotryptase is an endocellular distase. However, since it is capable of liquefying gelatin, it must be admitted that under certain conditions it can diffuse through the cell membrane (Will), especially when the yeast is in an abnormal state (dead or diseased cells).
The esterase group comprises, besides the fat enzymes (lipases), a series of other diastases involved in the saponification of esters, phosphoric acid (phosphatases), sulfuric acid (sulfatases), and the like. These different diastases are found in the yeast. They are reversible, that is, susceptible, according to the needs of the body, to cause the hydrolysis or synthesis of fats and esters.
The yeast lipase, which breaks down fats into fatty acids and glycerin, appears to be endocellular: it is involved in the use of fats found in protoplasm, particularly in the period of sporulation. In some yeasts, however, it is able to diffuse through membranes (Van Tieghem). This would be so in particular for various Torulas (Rogers and Jensen).
According to the nature of hydrolysed bodies, glucidases [glucosidase?] are divided into disaccharases or polyases, according to whether they transform disaccharides (sucrose, maltose, lactose and trehalose), trisaccharides (raffinose) or polysaccharides (starch, glycogen, inulin) into hexoses.
Amylase converts starch into maltose and appears to be formed by several distinct diastases. It is found in many species of mold (Aspergillus oryzae, Amylomyces Rouxii, etc.) and bacteria (butyric ferments, Bacillus mesentericus B. subtilis), but rarely in yeasts (Schizosaccharomyces Pombe, Schizosaccharomyces mellacei, Yeast Logos). Inulase, which produces levulose from inuine, is also found only exceptionally (Schizosaccharomyces Pombe and Mellacei, S. marxianus). On the other hand, yeasts contain an enzyme very close to the amylase, glycogenase, which saccharifies glycogen by first giving maltose, then glucose. This diastase is endocellular and therefore acts only on glycogen produced by the yeast itself.
Raffinose is split into levulose and melibiose by raffinase (diastase close to sucrase), and melibiose is itself broken down into glucose and galactose by melibiase. Some yeasts can ensure complete decomposition (bottom brewer and baker’s yeast, S. pastorianus, etc.); others attack raffinose but have no action on melibiose (Schizosaccharomyces Pombe, Schizosaccharomyces mellacei, Yeast Logos).
Sucrase or invertase, which hydrolyzes sucrose to glucose and levulose (invert sugar), is the most common of all diastases. It is found in many molds and most yeasts. Some of these, however, do not contain them (S. Ostosporus, S. apiculatus, S. Rouxii, etc.) and therefore can not ferment sucrose. In some species, diastase remains inside the cell, but in many cases it can diffuse to the outside. Sucrase prefers acidic media (optimum pH: about 5). It already acts at 0°, has the optimum temperature 52° and is very quickly destroyed at 70°. The most favorable saccharin concentration is 20%; invert sugar and levulose have a retarding effect on hydrolysis.
Maltase, which converts maltose into 2 glucose molecules, is found in various molds (Asperpillus niger, A. oryzae, Amylomyees, various Mucors) and in many yeasts. It does not exist in S. apiculatus, exiquus, marxianus, or Jorgensenii. It works best in a neutral medium (optimum pH 6.6) and at a temperature of 40°.
Lactase breaks down lactose into glucose and galactose. It is only found in a small number of yeasts (fermented milk yeasts). It is more prevalent in molds.
Trehalase, which gives from trehalose, glucose and levulose, has been found in many beer and wine yeasts (Kalantar).
Alcoholic zymase splits hexoses into alcohol and carbon dioxide. This diastase is a complex product, probably constituted by a mixture of various enzymes. Harden and Young have shown that it is constituted, at least, by a colloidal part whose fermentative proprieties disappear by heating, the apozymase; and by a non-colloidal heat-resistant part, cozymase. The latter seems to have the structure of a phosphorous organic ether: it would be, according to recent work, a magnesium adenylphosphate.
Zymase is very fragile. Abandoned to itself, the yeast juice rapidly loses its activity, as a result of the destruction of the apozymase by the proteins of the yeast and the hydrolysis of the cozymase by the lipase.
Zymase prefers an alkaline medium. Addition of carbonate and sodium phosphate has a favorable effect on the fermentative power of diastase. On the other hand, action of zymase is stopped by the sublimate at 0.1%, the alcohol at 15%, the formal [formaldehyde?] at 10%.
Diastase is destroyed by heating at 55°; in the dry state and if the desiccation was carried out under vacuum at 40°, it can however withstand up to 140°. The ferment power is maximum around 12-14° (Buchner). The optimum temperature appears to be higher, but protease attack increases as the temperature rises. The best sugar concentration is around 25%.
Autolysis of the yeast.
If quantity of yeast is less than 40% of the weight of the sugar, the fermentation stops frankly when the sugar is exhausted. But when the weight of yeast is more than 40% of that of sugar, the fermentation continues, the longer the amount of yeast employed is greater. The yeast then lives on its own substance: it is the phenomenon of autophagy or autolysis.
Two phases can be distinguished in autolysis: glycogen fermentation and proteolysis. Glycogenase contained in the cell acts on glycogene, which is fermented by giving alcohol, carbon dioxide, glycerin, and, according to Salkowski, succinic acid.
At the same time, nitrogenous materials are attacked by various proteases. Among the products of this digestion are nucleic bases, valine, leucine, guanine, lysine, arginine, aspartic acid and choline (Kutscher).
Autolysis is facilitated by the rise in temperature and a slightly acid reaction of the medium (optimum pH = 6). Some salts (acid phosphates, NaCl, acetates, citrates, arsenates also favor the phenomenon while others (neutral phosphates, nitrates, ammoniacal salts) delay or hinder it (Lintner).
Action of Physical and Chemical Agents
Temperature.
In general, when wet, the yeast cells are killed by heating for a few minutes at 50-60°. Some non-resistant species perish at 45-48° while others are only destroyed at 70°. Wills has found wild yeasts that can withstand heating at 70° for 30 minutes. Deadly temperature also varies according to the composition of the medium. Heat resistance is lower in acidic medium than in alkaline medium. It is larger in the presence of sugar or starch: thus, in bread, deadly temperature would be around 68°, according to Wills.
In spore state, yeasts support temperatures of 5 or 10° higher than those at which cells perish. Dry yeast resists much better than wet yeast: it can withstand a heating of 5 minutes in a current of air heated to 100°, and even 120° for certain species.
Cold has little effect on yeasts: cells have been cooled to 150 degrees below 0 in liquid air, without altering their vitality, provided that temperature changes are gradual and not abrupt.
Extreme temperatures at which fermentation can take place are close to 0° and 40°. Except for very rare exceptions, all fermentation ceases beyond 40-42°. Cochran and Perkins (1), however, studied yeasts which, heated in a 58° syrup for 30 minutes, continued to ferment the liquid.
(1) Int. Sug. J. XXXV, 26, 1933.
Optimum fermentation temperature is generally between 25 and 35° C. It varies according to the yeast races and composition of the environment. For distillery yeasts from tropical countries, it reaches 32-35°. It is also possible to acclimate races of temperate regions, whose thermal optimum is relatively low, to the high temperatures of hot countries (Chaturvedi, Owen).
Madams E. Bachrach and J. Roche have shown that prolonged action of potassium chloride causes a shift of thermal optimum to high temperatures. They found that after 10 months of culture on yeast water supplemented with a high dose of KC (10%), the yeast under study had its optimum cell multiplication temperature elevated 3°. The same fact was also observed with the lactic ferment (elevation of 5 to 6° after 3 years). (2)
(2) C. R. CXCIV. 1023, 1932
Optimum temperature zone is, on the whole, very close to that of deadly temperatures. On the other hand, toxic action of the components of the must which hinder yeast functioning (alcohol, organic acids, etc.), is found in general considerably increased by rise in temperature. As alcoholic fermentation is accompanied by a strong release of heat (20 to 23 calories per 180 grammes of sugar destroyed, according to (Bouffard), which raises the temperature of the liquid, we understand that certain fermentations begun at a temperature too high can stop abruptly in full operation.
It is often necessary, in breweries and wineries, to choose fermentation temperatures much lower than optimal temperatures, either to hinder development of certain foreign organisms or to give the fermented liquid particular characteristics from the point of view of taste. For example, in the preparation of beer by bottom fermentation, one goes down to 4-10° in the vat room and to 0.5-4° during resting in the cellars.
Light.
Light does not seem to have a very marked action. However, according to Lubimenko (1 [this may be a typo?]), it would slow cell multiplication. Fermentative energy as well as quantity of alcohol formed would be lower for the illuminated yeasts than for those which develop in the absence of light, the difference being all the more pronounced as the temperature is higher. The proportion of acids produced (especially volatile acids) would also be higher and that of glycerine less. According to Owen (3), a short exposure to ultraviolet rays would increase the speed and efficiency of the fermentation of cane molasses.
(3) Ind. Eng. Chem. VI, 480, 1914.
Oxygen.
In experiments that have remained famous, Pasteur has shown that yeast, sown in a sugar solution placed in a flat bowl, burns the sugar with carbon dioxide and water, reproduces abundantly and gives only traces of alcohol. Seeded on the contrary in a solution deprived of air by boiling and kept away from air, it multiplies much less than in the previous case, but produces only alcoholic fermentation. Pasteur concludes that: “fermentation is the consequence of life without air, without free oxygen gas … The aerobic being makes the heat it needs by the combustions resulting from free oxygen gas, the anaerobic being makes the heat it needs by decomposing a so-called fermentable material, which is of the order of the explosive substances likely to release heat by their decomposition”.
The Pastorian theory, after having been disputed for a long time by certain physiologists (Cochin, Brown), has received in recent years a striking confirmation, in particular following the work of Meyerhoff. [I think this is Otto Fritz Meyerhof, but I see his last name spelled differently sometimes.]
There is antagonism between yeast “plant function” and “ferment function”: proliferation of the cells is increased, but intensity of the alcoholic fermentation is reduced in the presence of air. This reduction is, however, quite variable depending on breeds of ferments: relatively small for yeasts with a low respiratory intensity (bottom fermenting brewer yeasts), it increases sharply when the respiratory power is very great (Torulas), as shown by following figures obtained by Meyerhoff:  CO2 (respiratory coefficient) indicates the number of mmc. of O consumed by mgr. dry yeast and per hour; *** the number of same, CO2, released by the same weight of yeast per hour in a 5% sugar solution, in the presence of air; *** the same amount of CO2, released under the same conditions, in a nitrogen atmosphere.
CO2 (respiratory coefficient) indicates the number of mmc. of O consumed by mgr. dry yeast and per hour; *** the number of same, CO2, released by the same weight of yeast per hour in a 5% sugar solution, in the presence of air; *** the same amount of CO2, released under the same conditions, in a nitrogen atmosphere.
The best yields are obtained industrially when the yeast is forced to live in anaerobic life, for example by operating in closed tanks under pressure of carbonic acid. A minimum of oxygen is however necessary as exciting cellular: Placed in a culture medium completely devoid of oxygen, the yeasts soon degenerate and die (Cochin, Brown). [cellular functions?]
Acids.
Yeasts prefer an acidic medium. Optimum pH, corresponding to the maximum development of cells, varies with the breed and conditions of culture. It is usually between 4.5 and 5.0. The optimum is close to neutrality, when growing conditions become unfavorable (temperature rise, environmental poverty, addition of antiseptics, etc.). Bacteria are much more sensitive to acidity than yeasts, especially putrefying bacteria, whose optimum pH range is between 6 and 7 and the minimum pH between 4.4 and 5 usually. In industrial technology, this difference in the sensitivity of enzymes is used to favor some of them.
Mineral acids act mainly as a function of the pH. The fermentation is stopped by 0.5% hydrochloric acid, 0.7% sulfuric acid, 0.75% phosphoric acid.
Some organic acids have a much more pronounced action. We shall say a few words of those most often found in the fermentations of cane molasses; formic, acetic, butyric, lactic, citric and oxalic acids.
Johannessohn (1) found that formic acid and its higher homologs, at very low dilution, accelerated alcoholic fermentation and that the optimum concentration of these acids was directly related to the molecular weight. This concentration would be 5.06 grams per liter, for example formic acid, 6.6 mg for acetic acid, and so on. If it increases, the fermentation is hampered and then stops. The amount of acid which completely stops the functioning of the zymase is, however, not sufficient to kill the yeast.
(1) Biochem. Z. XLVII. 97
According to Henneberg, a dose of 0.08% of formic acid would already weaken the yeast and a dose of 0.2% would stop its development. It is in the presence of this body that it is probably necessary to attribute some of the difficulties that one experiences in fermenting certain molasses, in particular those of the refinery where it has been found up to 0.791% of formic acid. (Zerban).
Different yeasts are unequally sensitive to the action of acetic acid. Meissner (2) found, for example, a 0.25% dose of this acid completely removed the Saaz and Frohberg yeasts [lagers], a dose of 0.375% yeast Logos, while 15 races of wine yeasts were able to finish the fermentation in the presence of 1% of acid. The harmful effects of acetic acid also vary, as Zikes has shown, depending on whether the acid is added to the must or is released during fermentation.
(2) Erlangener Dissert, Berlin 1897.
Yeasts are very susceptible to the action of citric and oxalic acids, which can be produced by molds and bacteria during molasses storage. Kayser found that the addition of 0.2 to 0.4% citric acid greatly slowed fermentation. According to Buromsky’s observations, the relative development in the presence of 1% of citric acid would be, for different yeasts: race XII (Berlin) 1.9, Logos 7.2, Frohberg 3.6. Saaz 1.4, S. ellipsoideus 21.3, S. pastorianus 10.3.
Oxalic acid, when its proportion is less than 0.052%, acts as a yeast stimulant, but at higher doses it is harmful and kills the cells in 2 hours at 0.4-0.5%. With Lebedeff [an old term for dried yeast?], the action is much more sensitive in pure sugar solutions: oxalic acid is already harmful at 0.001% and cells killed at 0.1-0.2%. Although the salts of oxalic acid are much less harmful than the free acid, oxalate shows however toxic at a dose of 0.25%.
Neale and Maerker found that butyric acid retarded yeast development at 0.05% concentration and stopped it completely at 1%. Müller, however, observed that a 0.5% dose of butyric acid slowed fermentation down only slightly, but much more strongly affected cell growth. This one would already be hampered by the low concentration of 0.005%, according to Juslin. Butyric acid, at relatively low doses produced during molasses storage which have been exposed to moisture, may therefore be the cause of lazy fermentations of certain musts.
Yeasts show, on the other hand, a high tolerance towards lactic acid, which is one of the most commonly used antiseptics in grain distilleries. Hayduck, for example, has observed that this body has a retarding action on yeasts only if it reaches a concentration of 1.35%. At 0.5%, it stimulates cellular development. This is so when the temperature remains relatively low. But if it exceeds 40° during fermentation, lactic acid can be very harmful and greatly reduce alcohol yield, this being partly due to increase in toxicity of the acid with temperature rise, but especially to the preponderance taken by lactic acid bacteria.
In Jamaica, Ashby has studied the action of acetic, lactic and butyric acids at various concentrations on budding yeast (Saccharomyces) and on fission yeast (Schizosaccharomyces) seeded in a cane juice mash and vinasse, having a density of 15° Brix and an initial acidity of 0.24% as SO4H2 [sulfuric acid]. He obtained the following results:  Alcohols.
Alcohols.
Antiseptic power of alcohols increases rapidly as the number of carbon atoms increases. Thus, Regnard observed that a solution of 2 gr. of glucose in 250 cc of water no longer fermented in the presence of: 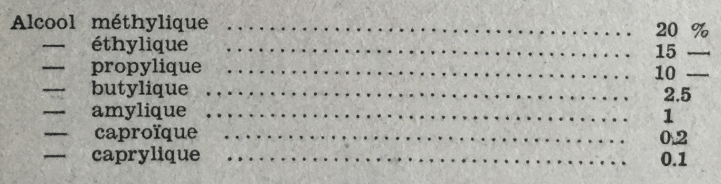 Various yeasts are very unevenly resistant to high alcohol concentrations. As soon as it reaches 6 to 8°, ethyl alcohol becomes antiseptic for some of them, while other breeds can produce 15 to 16% alcohol without being inconvenienced. Went and Geerligs have observed, for example, that Saccharomyces Vordermannii, found in Java distilleries, can rapidly ferment solutions containing 18-19% glucose. Inui has described, under the name of Saccharomyces Awamori [from a Japanese wine], a yeast whose activity begins to be hindered only by 13% alcohol and is completely stopped only from 20% alcohol. According to Gray (1) tolerance to alcohol would not be specific to the genus or species, but would depend solely on the yeast race.
Various yeasts are very unevenly resistant to high alcohol concentrations. As soon as it reaches 6 to 8°, ethyl alcohol becomes antiseptic for some of them, while other breeds can produce 15 to 16% alcohol without being inconvenienced. Went and Geerligs have observed, for example, that Saccharomyces Vordermannii, found in Java distilleries, can rapidly ferment solutions containing 18-19% glucose. Inui has described, under the name of Saccharomyces Awamori [from a Japanese wine], a yeast whose activity begins to be hindered only by 13% alcohol and is completely stopped only from 20% alcohol. According to Gray (1) tolerance to alcohol would not be specific to the genus or species, but would depend solely on the yeast race.
(1) J. Bacteriology XLII, 561, 1941.
The action of alcohol is dependent on temperature. Thus, Muller-Thurgau (1) noted, for the particular yeast he studied, that fermentation was stopped by amounts of alcohol below (by weight):
(1) In F. Falar – Technical Mycology. London, 1898-1910.  Antiseptics.
Antiseptics.
Under the name of antiseptics, are designated substances which hinder the development of ferments. Depending on the dose used, they can paralyze certain vital phenomena (reproduction, fermentation, etc.) or cause complete cell destruction. At low doses, they interfere with the multiplication of yeasts and increase the ferment capacity.
The mode of action of antiseptics is very variable. They can act as oxidants (K permanganate, hypochlorites), as hydrolyzers (strong acids), by coagulating protoplasmic colloids (phenols, creosote) or by forming with them combinations of absorption (corrosive sublimate, heavy metals), etc.
Antiseptic action depends not only on the nature and origin of microbes or yeast, but also on many other factors: temperature, reaction, chemical composition of the medium, and so on. It is increased by the rise of the temperature, acidity of the must and its poverty in nutritive matters. Biernacki indicates, for various products, concentrations accelerating and preventing the fermentation:  Yeasts may be accustomed to withstanding increasing doses of anti septic. They thus acquire a new physiological state, which can persist even after a series of cultures and be manifested by a variation in the composition of the products formed. In the case of acclimation to fluorides, for example, the yeast is enriched more and more in mineral substances: the lime that appears inside the cell insolubilizes fluorine, in the form of lime salt. There is a reduction in the amount of glycerine and succinic acid formed.
Yeasts may be accustomed to withstanding increasing doses of anti septic. They thus acquire a new physiological state, which can persist even after a series of cultures and be manifested by a variation in the composition of the products formed. In the case of acclimation to fluorides, for example, the yeast is enriched more and more in mineral substances: the lime that appears inside the cell insolubilizes fluorine, in the form of lime salt. There is a reduction in the amount of glycerine and succinic acid formed.
The most commonly used antiseptics in the fermentation industries are sulfur dioxide (vinification), hydrofluoric acid and fluorides (distillery), dilute sulfuric acid, lime milk, bisulphites, formalin (cleaning of the vat room).
Toxins.
Hayduck first reported in 1909 the existence in yeast of an endotoxin capable of killing it, when extracted from the cells and introduced into the culture medium.
Ferbach (1) confirmed this existence and observed that the toxic substance seems to play with regard to yeast, and also with respect to bacteria, the role of an antiseptic. Yeast endotoxin shares with some known toxins the property of passing through porcelain filters and of being destroyed at a temperature of 100°. But it is clearly distinguished by its volatility: it is easily entrained with water vapor, when distilling toxin macerations under reduced pressure, so as not to exceed 40° (Fernbach).
(1) C. R. CXLIX, 437, 1909.
Under certain conditions, the toxins produced by yeasts could diffuse into the culture liquid and prevent a new development of the ferment in this medium (vaccinated or immunized solution). Boulard (2) has indicated a method for obtaining this result with fermented beverages.
(2) C. R. CLXXXIII, 1422 1926
“With a liquid, such as a wine must, for example, containing 250 grammes of sugar per liter, a first fermentation is determined by the usual method, with one or more yeasts, and the fermentation is well declared, and that 20 or 30 grammes of sugar have been transformed into alcohol, the liquid is heated for about an hour, at a temperature a few degrees higher than the deadly temperature of the yeasts which are in the wine. As a general rule, it suffices to reach 45° C. The liquid is then cooled, then brought back to the optimum temperature of fermentation, it is once again sown with the same yeasts, then when the fermentation is again clearly declared, it is heated a second time as it has been previously. In general, it suffices for 3 operations of this type to stop all fermentation and to render the liquid unfermentable even after the addition of a significant amount of yeast and while the dose of unfermented sugars is still greater than 150 gr. per liter.”
This phenomenon appears moreover very general, and it would be possible, by this method, to vaccinate any liquids before their complete transformation and to prevent their subsequent invasion by a certain ferment (Boulard).
Longevity of the yeasts.
Yeasts are likely to remain in the same environment for a long time without perishing. Duclaux, examining old Pasteur cultures after 11 to 17 years, observed that out of 26 yeasts, only 6 could not be revived. Klöcker found that living cells were found in sucrose and beer must solutions after 20 and 30 years. Finally, Gayon and Dubourg examined wines made in 1810, 1818, 1819, 1832, 1836 and 1846 and found that they still contained yeasts capable of provoking alcoholic fermentation.
The storage faculty also depends on several factors: race and origin of the yeast, presence or absence of light, culture medium, etc. According to Will, wild yeasts have a more prolonged vitality than those of culture, some of which can die very quickly. Sunlight seems pretty damaging to conservation. It would be yeasts living aerobically on the surface of liquids that would have the greatest vitality, according to Henneberg.
Culture, isolation and examination of yeasts
Culture methods.
Classical methods of bacteriology are used for culturing yeasts. The necessary equipment are: test tubes, Roux tubes, Petri dishes, Erlenmeyer flasks, Chamberland autoclave, etc. and, for physiological research, the balloons of Pasteur [glass balloons], Chamberland, Feudenreich, the flasks of Hansen, etc.
However, unlike bacteria, yeasts require a slightly acidic reaction. Culture media should be placed in thin layers, so as to achieve the maximum aeration, if we want to obtain the multiplication of cells; and, on the contrary, in deep vials, if one wishes to provoke the alcoholic fermentation. The most commonly used liquid media are:  This solution has been used by Pasteur in most of his studies on alcoholic fermentation.
This solution has been used by Pasteur in most of his studies on alcoholic fermentation.  Any fermentable sugar may be added to this solution, which has been used by Laurent in his work on the hydrocarbon nutrition of yeasts. Pairault advises, for the study of rum yeasts, the following solution:
Any fermentable sugar may be added to this solution, which has been used by Laurent in his work on the hydrocarbon nutrition of yeasts. Pairault advises, for the study of rum yeasts, the following solution:  Filter if necessary and sterilize for 20 minutes in an autoclave at 120°. The formula of the special nutrient mixture is: Am phosphate: 100, K sulfate: 60. Mg sulfate: 10, Ca acid phosphate: 30.
Filter if necessary and sterilize for 20 minutes in an autoclave at 120°. The formula of the special nutrient mixture is: Am phosphate: 100, K sulfate: 60. Mg sulfate: 10, Ca acid phosphate: 30.
Culture media are also often used: beer must and malt extracts; fruit juice; decoctions of carrots, potatoes, etc .; yeast water. The latter is prepared by boiling 100 gr. fresh yeast in 1 liter of distilled water; it is filtered and sterilized. To obtain a high proliferation of cells, it is important to add sugar.
As solid media, slices of potato, turnip, carrot, etc., or beer, fruit juice, with 8% gelatin or 1.5% of agar can be used.
Study of yeast sporulation requires a special technique. First of all, cells must be young and well-nourished, which is obtained by growing the yeast, for about 48 hours, in a nutrient medium (beer must, for example) with repeated transfers. Then, the cultures are subjected to a period of starvation, by seeding on block of plaster or in a not very nutritive medium.
In the Engel-Hansen method, a conical or cylindrical plaster block is prepared by mixing plaster of Paris with 3 parts of water. The block is placed in a glass box, at the bottom of which we put a little distilled water or beer beer, up to about half of the height of the block. The box is covered with a lid, without it preventing the free flow of air. To reduce the risk of infection by bacteria, it is advantageous to replace the glass box with a Hansen bottle. After the apparatus has been sterilized, by heating at 115° C. for 1/2 hour, the yeast is deposited on the plaster using a sterilized platinum wire [inoculating loop], and the oven is heated to room temperature, more favorable for sporulation (25-30°). After about 100 hours, most cells formed ascopores.
In the Gorodkowa method, simpler than the previous one and which gave excellent results to Guilliermond, a gelatin medium is seeded with young and vigorous yeast, having the following composition:  Yeast develops vigorously after plating, but budding stops soon, due to the small amount of sugar available. After 2 to 3 days, sporulation is complete [spores may be thought of as the seeds of yeast?].
Yeast develops vigorously after plating, but budding stops soon, due to the small amount of sugar available. After 2 to 3 days, sporulation is complete [spores may be thought of as the seeds of yeast?].
Many other media have been used to obtain spore formation; yeast water, gelatin or nutrient agar, carrot slices, etc. Carrot culture is particularly indicated for cytological studies (Guilliermond). Most yeasts, especially those of the genus Schizosaccharomyces, produce ascospores in this medium after 6 to 8 days and sometimes in less time.
Purification and isolation.
The principle of the methods currently applied to purify and isolate yeasts is to dilute the liquid containing the microorganisms, so that there is no more than one cell in a given volume of the culture medium. [There is a beautiful demonstration of this in Lallemand’s Alcohol Textbook (6th edition) from the head distiller at Jack Daniel’s isolating lactic acid bacteria.]
Hansen’s method. — First, dilute the yeast mixture with distilled water. A drop of liquid is taken and the number of cells found under the microscope is determined by means of a grid glass. Then dilute so that there is only one cell in each second drop. During these manipulations, it is important to strongly agitate the contents of the flasks, to have a complete separation and a uniform distribution of the cells in the dilution water. A droplet of liquid is then deposited in a series of flasks containing sterile must and the flasks are left on their own until colonies have developed on the bottom of the containers.
Balloons showing a single yeast spot contain a pure culture, coming from a single cell.
A second method, devised by Koch and perfected by Hansen, uses solid media, gelatin or agar. The yeast mixture is first diluted in distilled water and, after counting the cells, a drop is deposited in a suitable volume of gelatinized must at 30° C. The mixture is stirred well to separate the microorganisms and spread a drop of the mixture on a grid slat [streaked]. This is placed in an ordinary wet chamber or, better still, a Bottcher wet chamber (also called Van Tieghem and Lemonnier cell), which can be examined under a microscope at any time. It is easy, therefore, to follow the development of the colonies by regular microscopic observations. When the observer perceives a well isolated germ, he marks it by the pointer or by inscribing the square dummy number of the grid slat. It only remains to transfer the colony to a liquid medium, after sufficient development. [I think these are now called perfusion chambers.]
A disadvantage of culture in a solid medium is that the food reaches the cells only by diffusion and so slowly that they can die before their complete development.
Lindner method. — Lindner, after having diluted the yeast (in a beer must, for example), so that the liquid contains only one cell per drop, successively touches a certain number of points of a sterile Petri dish with the liquid, which was introduced into a flamed pipette with a fine opening. Wherever there will be a single cell, a single spot will be formed. It is then easy, using platinum wire, to transport the colonies in a nutrient medium in order to multiply them.
Another method, known as the Lindner droplet method, consists in depositing, by means of a sterilized metal pen, small droplets of the dilution of yeasts, in more or less tight lines, on a slide which we return to the humid chamber to allow microscopic examination. [These now may be called perfusion chambers and have inlets, outlets, and temperature regulation for live cell observation.]
Lindner’s method is simpler than Hansen’s, but less reliable. The microscopic examination aiding, it gives however very good results.
Yeasts examination.
Cell counting.— The yeast cells, which are suspended in water contained in a very small container of known dimensions, are counted directly by means of a microscope. [hemocytomer] The apparatus generally employed is constituted by a slide on which is fixed, with putty, a coverslip of 0.2 mm. thick, in the middle of which we made a circular opening. It is closed at its upper part by a slat applying exactly on the bowl. The object is equipped with a micrometric grid, Each square of the network is 0.05 mm on each side and forms the basis of a prism with a volume of 0.0005 mmc. It is also possible to use a hemocytometer constructed for counting blood cells. After having diluted and agitated the wort strongly, so as to obtain a homogeneous distribution of the yeasts, a drop of liquid is deposited in the bowl of the apparatus, which is covered with its slat, avoiding imprisoning an air bubble. A few minutes are waited for the cells in suspension to settle and the cells are counted (at a magnification of 300) in a series of squares placed next to one another. A second preparation is then carried out, on which the same procedure is followed and continued until the average number of cells contained in 5 squares, for example, no longer varies significantly.[I recently wrote about this and collected the best modern tips and tricks for success.]
Yeast development. — It is easy to follow the development of yeasts under a microscope. After having diluted the culture medium, so that a drop of it contains only a small number of cells, a droplet of liquid is placed on a coverslip and placed in a humid Bottcher chamber. The yeast cells can thus be stored for a period of 8 days without danger of contamination and follow the phenomena of budding, sporulation, spore germination, etc. [perfusion chamber]
To observe development of ferments at different temperatures, small ovens were built (Ranvier, Vidal, etc.), which fit on the platter of the miscroscope and which are maintained at the desired temperature by a regulator. But, most often, we can do without these devices, putting the cells in an ordinary oven during the interval of operations. [employing some of these ideas may be useful because Pombe yeast can operate at higher temperatures than other yeast which may be key to their dominance.]
Examination of spore germination presents some difficulties, as there are still some cells in the preparations which do not sporulate and which develop before ascospores. This disadvantage can be overcome by operating as follows. A small portion of yeast culture, made on solid medium (gelatin, carrot, etc.), is extended by means of a spatula on a sterile slide, and this is placed in an oven at 55-60° for 12 hours. Vegetative cells are killed and only ascopores survive. The mixture is then wetted with a little water, and a drop is placed in the humid chamber. It is also possible, as advocated by Hansen, to treat yeast with absolute alcohol or 50% alcohol; vegetative cells are killed in one minute, while ascopores resist for a long time. [It is still unclear at this point why you want to isolate the spores. If they are like a seed, are they the healthiest thing to preserve and/or regenerate the population?]
Microscopic reactions. — In general, living yeasts do not take the usual dyes. However, treated with neutral red (in aqueous solution at 1:10,000), the metachromatic corpuscles are slightly tinted, while the nucleus and protoplasm remain colorless. Dead yeasts easily stain with 0.5% methylene blue and gentian violet.
[This 0.5% number implies you would be buying 1.0% methylene blue (commonly sold that way) and using it for your final 2 fold dilution. I did come across more modern, less toxic, staining ideas in my cell counting post.]
To study the nucleus, fixation is first carried out by means of Bouin picroformol solution or Perenyi solution, and then stained with Heidenhain’s ferric haematoxylin. It is also possible, after fixation in Bouin solution, to use the Delafield hematoxylin method.
The yeast membrane is stained, after fixation, with Ehrlich’s methylene blue or Hanstein’s aniline.
Tincture of iodine (Lugol’s solution) stains glycogen in red-brown, and osmic acid (Flemming solution) in yellow or blackish-brown fat globules. To distinguish the latter from oil droplets, which are often found in the cells of the film yeast, it is treated successively with alcohol and concentrated sulfuric acid: the oily droplets then take on a greenish-gray hue, which finally becomes blackish-brown.
Physiological properties. — To determine resistance to acidity, one can use the very simple method of Duclaux. It consists in inoculating, with the yeast, tubes of sweet wort acidulated with 1, 2, 3% of tartaric acid. The time elapsed between seeding and the appearance of a disorder, due to the development of the yeast, is noted.
The optimum temperature of fermentation is determined by seeding the yeasts in a must rich in sugar, to be sure that unfermented sugar will remain, and by heating in an oven at various temperatures, kept constant (23°, 30°, 35°, etc.). After about ten days, the remaining sugar is measured. A similar procedure is used to determine the ability of the yeast to ferment musts rich in sugar. A must containing 30% glucose is inoculated and the remaining sugar is measured after fermentation.
The ability to ferment various sugars is a very important trait for the distinction of yeasts. This property can easily be appreciated by means of Lindner’s method. A drop of aqueous solution of yeast is placed in a regular moist chamber with the help of a platinum wire, and a small quantity of sugar to be studied, which has been pulverized beforehand, is added. Cover with the coverslip, whose sides are closed with a little Vaseline, and take to the incubator. By examining the microscope preparation the next day, the appearance or absence of carbon dioxide bubbles indicates whether the sugar is attacked by the yeast. To make sure that it is CO2 bubbles, we can drop a few drops of caustic scale on the pore-object: the CO2 bubble contracts and disappears. It is essential to make the seeding with an imperceptible trace of yeast otherwise, the glycogen contained in the cells could give rise to a release of carbonic acid.
It is also possible to seed the yeast to be studied in invert sugar solutions of sucrose, etc., and to follow, with the aid of the polarimeter, the variations of the polarimetric rotation of the liquid. We are thus aware of the more or less rapid disappearance of the C6 sugars from the speed at which the C12 sugars are exchanged, and so on.
The determination of the ferment vigor and yeast activity, which are of special importance from an industrial point of view, is carried out by different methods. The dosage of alcohol or sugar can be carried out during the fermentation process, but this has the disadvantage of requiring a lot of time, if many determinations are necessary. It is more convenient to measure, volumetrically or gravimetrically, the quantity of carbonic acid released.
Volumetric dosing requires use of a special apparatus to collect all the gas produced during the fermentation: we can use a regular nitrogen, which is filled with mercury, to avoid the absorption of gas which is would produce with water or other liquids. More simply, we can content ourselves with making successive weighings of the fermentation tank, equipped with a special closure allowing the free release of carbon dioxide and retaining the entrained water vapor, the Meissl valve or the Alwood valve, for example, which force the gas to bubble into sulfuric acid before escaping into the atmosphere. [very clever!]
In commercial yeast testing, we dilute 5 gr pressed yeast (or 50 g of liquid yeast) with 400 cc of 10% sugar solution in distilled water. It is introduced into a fermentation flask, closed with a fermentation closure and the flask is weighed. This is then placed in a water bath or oven rigorously set at 30° C. After 24 hours, we weigh again and the weight loss in gr of carbonic acid represents ferment power. [This will come in handy! I learned a ton about this and its math while studying the Champagne method.] The activity, or impulsive power, of the yeast is determined as follows, by the method of Meissl. A small quantity of yeast (1.0 gr) Is weighed and diluted with 50 cc of sugar solution, to which nutrient salts (1) have been added, in a small fermentation flask. Everything is weighed. The apparatus is placed for 6 hours in an oven at 30° C. and, after passing a stream of air to expel carbonic acid which is still in the flask, weighed again. Weight loss, calculated in gr for 100 gr of yeasts, is the number that measures impulsive power. Meissl calls normal yeast the one which, under these conditions, equals 1.75 gr of CO2, and gives it the value of 100. [Again very clever, and these days I think we may de-gas ultrasonically]
(1) The sweet solution consists of 400 gr refined sugar, 25 gr monoammonium phosphate and 25 gr mono-potassium phosphate. Dissolve 5 gr mixing in 50 cc well water.
Yeast preservation.
Hansen’s work has shown that yeasts can be stored for a long time in 10% pure sucrose solutions. Most species can survive for periods ranging from 15 to 17 years. Holm recommends the following solution:  A Hansen vial is usually used to preserve the yeast.
A Hansen vial is usually used to preserve the yeast.
Another method, advocated by Will, is to dry the yeast, so that it contains only 15 to 20% water, and to mix it with pulverized silica, plaster of Paris and coal. The mixture is dried at 40° and placed in hermetically sealed containers. By this method, it was possible to keep for 9 years some yeasts. During this period, the yeasts form ascopores (Hansen).
It is prudent to regenerate the yeasts kept in the laboratory from time to time (every month for example), by reseeding in new tubes of sterile sweet medium. [We see a great modern description of this in the Alcohol Textbook 6th edition regarding Jack Daniels.]
Identification and classification.
Polymorphism and variability of physiological yeast properties make characterization of species identification difficult. Thanks to Hansen’s work, we can, however, succeed in differentiating these with a very satisfactory precision. Hansen used as distinguishing features: the shape and dimensions of cells at different temperatures and in different environments; the shape of the ascospores and their mode of germination; the optimal and extreme temperatures of budding, sporulation and haze formation: the appearance of the film and cultures on solid media (agar, gelatin); biochemical properties, and more particularly action on the different sugars. Lindner added: the appearance of “giant colonies”, obtained by inoculating a large plate of gelatin in its middle.
The most important characteristics are temperatures at which films and ascospores form. Also, when a yeast does not produce a film or give spores, its identification becomes much more delicate, if not impossible. [We probably need to learn more about film in this context versus other film yeast phenomena.]
We give below the classification of Hansen, modified by Guilliermond. Yeasts are divided into two families: Saccharomycetaceae, or true yeasts, which form ascospores – and non-Saccharomycetaceae, or non-yeasts, which do not give spores.
True yeasts.
They are subdivided into 5 groups:
1st group. — Yeasts with cylindrical cells, rectangular or oval, multiplying by transversal partitioning [fission]. Ascus with 4 or 8 ascopores, generally resulting from isogamic conjugation. Yeast vegetating on must of beer, form a deposit. This group contains only one genus: Schizosaccharomyces. [Ascus: a sac, typically cylindrical in shape, in which the spores of ascomycete fungi develop.]
2. group. — Yeasts multiplying by budding. Ascus from a conjugation (sometimes rudimentary). This group includes the following types:
Zygossaccharomyces: ascus resulting from heterogamic copulation; ascospores with a thick, smooth membrane;
Debaromyces: ascus resulting from a coupling usually heterogeneous; globular ascospores, with a verrucous membrane;
Nadsonia: ascus derived by budding from a cell formed by heterogamic conjugation; verrucous membrane ascospores more or less thick;
Schwanniomyces: traces of conjugation; ascospores with verrucous membrane, formed of two unequal parts separated by a salient ring;
Torulaspora: rounded cells, resembling those of torulas, with a large globule of oleaginous in the center; traces of conjugation in the formation of asci.
3. group. — Yeasts multiplying by budding, forming in the sugary solutions first a deposit, then a more or less mucous film, without occlusions of air; smooth, round or oval ascospores with 1 or 2 membranes, germinating by budding. Usually produce alcohol.
This group includes the following types:
Saccharomycodes: cells dividing by an intermediate process between bourgeonnement [budding] and scissiparity [fission], ascospores with a single membrane, germinating in the form of a tube;
Saccharomycopsis: ascospores with two membranes, germinating by budding;
Saccharomyces: round, ovoid, ellipsoid or oblong cells, ascospores with a single membrane, germinating by budding, sometimes with rudimentary mycelium formation;
Hansenia: apiculate cells, that is to say, provided at one of their extremities or both of a small point, which makes them resemble a lemon; hemispherical ascospores hat-shaped, with prominent rim.
The genus Saccharomyces, the most important from the point of view of fermentation industries, is subdivided into six subgroups:
a) Yeast fermenting dextrose, maltose and sucrose, but not lactose: S. cerevisiae, carlsbergensis, pastorianus, intermedius validus ellypsoideus, turbidans, willianus, Vordermanii, sake, etc.
b) Fermenting yeasts of dextrose and sucrose, but not maltose and lactose: S. marxianus, exiguus, mandshuricus, Zopfii, coreanus, etc.
c) Yeasts fermenting dextrose and maltose, but not sucrose or lactose: S. Rouxii Soja, Lindnori, Mangini, Chevalieri, etc.
(d) Yeasts fermenting dextrose but not maltose, sucrose or lactose: S. mali Duclauxi, unisporus, etc.
e) Yeasts fermenting lactose: S. lactis, fragilis, etc.
(f) Yeasts which do not produce alcohol and whose fermentation characteristics are little known: S. conglomeratus, theobromae, etc.
4. group. — Yeasts with budding, forming from the beginning, on sweet medium, a dry and opaque veil, with occlusions of air. Characteristic ascospores, provided with a kind of membrane and often with a projecting rim. Most of the species in this group do not give alcohol, but produce aromatic esters. The group includes the genres: [I wonder if Suaveolens is in here as a non ethanol producer? At this point in history, I think it was still classified as a mold.]
Pichia: often cylindrical cells; hemispherical, irregular or angular ascospores; mycelium rudimentary, fairly developed;
Willia: Ascospores in the shape of a lemon or hat, with a rim or a protruding ring.
5th group. — budding yeasts whose affinities are poorly known. Fusiform ascospores. This group includes the following types:
Monospora: ascus with a single needle-like ascospore, germinating laterally by budding;
Nematospora: ascus with several fusiform ascospores, terminated by an “eyelash” [un cil].
Non-yeast.
This family, which groups all yeasts not forming ascospores and whose place in the classification is uncertain, includes the following genera:
Torula: Generally spherical cells, often with a large globule of oil in the center. Species of this genus very often form a film (or in some cases a ring), but only after fermentation. The films are always viscous and without air bubbles. A number of torulas contain red or pink red pigments, more rarely black or brown.
Pseudosaccharomyces: Apiculate-shaped cells.
Mycoderma: Cells most often elongated, cylindrical, tending to remain united in thin chains. They form from the beginning of the culture on must of beer as folded films, filled with bubbles of air. Some species contain a red or pink pigment. Mycoderms normally vegetate on contact with the air and without giving alcohol. [I have observed these on dudner]
Cryptococcus: Yeasts resembling Torulas, parasites of humans and animals.
Yeast-shaped mushrooms.
They include species belonging to the most diverse groups. The most interesting from the point of view of the fermentation industries are the genera Endomyces, Monilia, Oidium.
Endomyces. — Mushrooms forming, on the basis of beer, from the beginning, a thick, fluffy film consisting of a typical mycelium, disarticulating into arthrospores. The cells from the budding of the articles of the mycelium give asci containing 4 ascospores.
Oidium. — Mushrooms with the same characteristics as the previous ones, but never producing asci.
Monilia. — Vegetable mushrooms on beer, in the form of a mycodermitic film, more rarely a ring, first composed by yeasts, then a typical mycelium, giving rise by lateral or terminal budding to yeasts and never producing asci. Sometimes the mycelium disarticulates into arthrospores.
The most important yeasts from point of view of fermentation industries belong to the genera Saccharomyces, which includes most crop breeds, and Schizosaccharomyces, which includes some interesting yeasts from hot countries. The following are the main species involved in the fermentation of sugar cane products.
Principal rhummerie yeasts
Schizosaccharomyces Pombe Lindner. Cells generally rectangular, rounded at the ends, measuring 7 x 4.5 mus [μ] on average. They multiply by partitioning, the transversal partition dividing the cell into unequal parts. Under certain conditions (absence of air), the cells lengthen a lot and can have many transverse partitions, without separation of the elements. Sometimes lateral branches are formed. The cells do not contain glycogen.  Sporulation occurs easily on slices of carrots (after a few hours), in old gelatin cultures and in musts after fermentation. Ascus shaped dumbbells. Ascopores 4 in number, arising in pairs in each bulge of the ascus, measuring about 4 mus of diameter and presenting on the surface of their membrane an amyoid substance which is colored blue by the iodine.
Sporulation occurs easily on slices of carrots (after a few hours), in old gelatin cultures and in musts after fermentation. Ascus shaped dumbbells. Ascopores 4 in number, arising in pairs in each bulge of the ascus, measuring about 4 mus of diameter and presenting on the surface of their membrane an amyoid substance which is colored blue by the iodine.  This yeast does not form a film on must of beer; but it produces a ring after a month. On gelatin, it gives a compact layer of fine flutes, with liquefaction of the medium. Beijerinck has so far reported the existence of a sporogenous variety, forming white colonies on gelatin and an asporogenous variety, producing brown colonies on the same medium. Schizosaccharomyces Pombe is a high attenuation surface yeast [top fermenting?], causing a vigorous fermentation. Minimum temperature 25° C; optimum temperature 30 – 35° [77°F, 86°F-95°F]. It ferments glucose, levulose, sucrose, maltose, raffinose, inulin and dextrin.
This yeast does not form a film on must of beer; but it produces a ring after a month. On gelatin, it gives a compact layer of fine flutes, with liquefaction of the medium. Beijerinck has so far reported the existence of a sporogenous variety, forming white colonies on gelatin and an asporogenous variety, producing brown colonies on the same medium. Schizosaccharomyces Pombe is a high attenuation surface yeast [top fermenting?], causing a vigorous fermentation. Minimum temperature 25° C; optimum temperature 30 – 35° [77°F, 86°F-95°F]. It ferments glucose, levulose, sucrose, maltose, raffinose, inulin and dextrin.
It was discovered by Saare and Zeidler, in a millet beer made by the natives of tropical Africa, the dollo or pombé. It has sometimes been used successfully for the production of pure yeast rum (Arroyo).
Schizosaccharomyces mellacei Jorgensen. A species very close to the preceding one, of which it is distinguished by its somewhat larger cells (1) and by the property it possesses of fermenting mannose, on which the yeast Pombé has no action. It is a yeast with high attenuation limit, giving good yields in alcohol and supporting a relatively high acidity. However, it is less active and ferment sugars more slowly than most Saccharomyces.
(1) Guilliermond observed that, grown on slices of carrot, the cells of Schizosaccharomyces Pombe measured about 7 m long and 4.5 m wide and those of Schizosaccharomyces mellacci 9.5 x 5 mus.
Isolated from molasses and vinasses sent in 1893 from Jamaica to the Jorgensen laboratory in Copenhagen, Sch. Mellacei was later studied by Greg, Allan and Ashby. [Search through the blog and you will find all of their papers.]
Greg pointed out the interest shown by this yeast, which gave him at the same time excellent alcohol yield, a very aromatic rum. But the duration of the fermentation, from 3-6 days in the case of budding yeasts, was increased to 12 days (must at 21° Brix).
Allan has found that in Jamaica it is almost the only yeast found in distilleries producing grand arôme rum. In the other rhummeries, we find, in roughly equal proportions, budding breeds and those with fission. The former usually predominate at the beginning of the rhummière campaign, often being supplanted after a while by the second, when the acidity of the musts increases.
Ashby isolated several races of Schizosaccharomyces. Most of them are top fermenting yeasts, joined in chains of 4 cells or more, and forming on the surface of the musts a brownish white hat. Some, however, are bottom-growing, showing isolated or 2-folded cells (2). The latter ensure a faster fermentation (6-8 days instead of 8-10 days, in laboratory tests of the author). The attenuation limit is about the same in both cases, but the bottom yeasts give a proportion of alcohol a little stronger than the high yeasts, which produce more esters and dry matter. [Ashby showed there were both top and bottom fermenting varieties. The top fermenting were superior for aroma.]
(2) These low yeasts could under certain conditions become high yeasts. Ashby has, indeed. observed that one of the yeasts isolated by him originally produced a low fermentation, but that after being kept for 2 months in a fermented and rejuvenated cane juice, it behaved like high yeasts.
With respect to alcohol resistance, Ashby found that fission yeasts examined by him could produce 12-14% alcohol, but only after a prolonged period of time (20-24 days). During the first 7-9 days, during which 9-10% alcohol is formed, the fermentation is regular and relatively fast, after which it slows down considerably. The doses of alcohol that hinder and stop activity of top fermenting yeasts are 8.5 and 12.5% respectively, while for bottom fermenting yeasts they reach 9.5 and 14% by volume. In practice, a concentration of 16% of sugars is the maximum limit for fermentation to be completed after a reasonable lapse of time (10-12 days).
Schizosaccharomyces are much more tolerant of acidity than Saccharomyces. While budding yeasts see their activity stopped by 1% acetic acid or 0.15% butyric acid, fission yeast are barely hindered.
On the other hand, when the medium is not very acid, the budding yeasts are more active and quickly take possession of ferment. Also, in vesou musts [fresh juice], whose acidity is generally less than 0.5%, only budding forms are found. In contrast, fission yeasts prevail in the mash of acidic molasses and are practically the only ones present when the acidity exceeds 1%. [1% is the rule of thumb, but I currently have a ferment seeded with both fission and budding yeasts where budding are dominant. 1% titrateable acidity does not exactly put you below pH 4.0 and many winery ferments start with pH of 3.6 or 3.8. It can take multiple iterations of dunder reuse and possibly the direct addition of vinegar to get a molasses pH below 4.0 without the addition of sulfuric acid (or ammonium sulfate).]
According to Cousins, Schizosaccharomyces would be conveyed by air, because we see them appear suddenly in distillery musts, where it is not possible to meet them at the beginning of the rhummière campaign. [I have never seen this note from Cousins. What I think was learned later is that they had a far lower frequency of occurrence than saccharomyces yeast.]
N. Deerr also isolated from a molasses of Peru a Schizosaccharomyces, with elongated cells, sometimes club-shaped, measuring 7.5-12 mus X 3.5-4.5 mus, ascospores often irregular, 4 in number of ascus. This yeast had some differences with the Schizosaccharomyces Pombe and Schizosaccharomyces mellacei.
Pairault observed that fission yeasts dominated at the beginning of the century in the molasses rums of Martinique and Guadeloupe. “This yeast,” he writes, “is found exclusively in our West Indies molasses cuvées, especially when the temperature is exceptionally high.”
According to Kayser, Schizosaccharomyces of the French West Indies differ from Schizosaccharomyces Pombe and Schizosaccharomyces mellacei because they do not have the property of fermenting dextrin. The same author has pointed out, moreover, the existence of several races (or species), differentiating among themselves by the shape and the dimensions of the cells, the appearance of giant colonies, etc. It describes in particular the following four forms:
Yeast IV (Martinique): Schizosaccharomyces branched and elongated 9.1 to 20 mus of length over 2.1 to 3.6 wide. Giant colony small, slightly discoid, slightly elevated center, a little craterform.
VI (Martinique): unbranched Schizosaccharomyces, very long and uniform; 11.7 to 18.2 mus long on 1.8 to 3.6 wide. Colony slightly scalloped and domed, yellowish in color, shiny appearance.
IX (Guadeloupe): Schizosaccharomyces with rectangular globules; 8.5 to 14 mus long and 2.2 to 2.7 wide. Colony with slightly elevated center, with scalloped, yellowish lobes.
XII (Guadeloupe): Schizosacchraromyces short, branched, 7.5 to 14.5 mus long on 2.7 to 4.5 wide. Colony similar to that of VI.
Kayser has, moreover, reported an interesting property of fission yeasts: that of giving substantially less higher alcohols than budding yeasts.
Schizosaccharomyces asporus Eijkmann. [Eijkmann was the nobel laureate that discovered vitamin B and worked in Batavia.]
This fission yeast was encountered by Eijkmann in arrack distilleries in Batavia. It is different from Schizosaccharomyces Pombe by the fact that it does not produce endospores. Beijerinck regards it as an asporogenous variety of the latter species. According to Groenwege, it is the main agent involved in the fermentation of Batavia arrack. It supports very high sugar concentrations, the density of musts in arrack distilleries being about 1.130 (27° Brix). Note that Ashby, in Jamaica, was also fermenting with the Schizosaccharomyces mellacei mash of molasses and vinasse, having as density 30° Brix and containing 23.3% of sugar at the beginning.  Schizosaccharomyces formosensis Nakazawa.
Schizosaccharomyces formosensis Nakazawa.
Ellipsoidal or irregular cells, measuring 9.2-16.8 mus by 4.8 mus; ellipsoid ascospores without glycogen, but with membrane impregnated with amyloid. The yeast forms on a beer must a film at 25-27° and a ring at 25-37° [I think that is brix, not temp]. It fermented glucose, levulose, sucrose, maltose, galactose, mannose, raffinose, inulin and dextrin. Optimum temperature of propagation: 32° C. [89.6F]
This yeast was isolated from sugary products in Formosa by Nakazawa, who found in the same habitat two other fission species; Schizosaccharomyces sautawensis Nakazawa and Schizosaccharomyces Nokkoensis, whose characters are not very different from those of Schizosaccharomyces formosensis.
Zygosacharomyces major Takahashi et Yukowa.
Spherical cells of 3.7-7.5 mus of diameter, sometimes oval, containing glycogen. Spores transparent, round or ovate, 3-4.5 mus of diameter in general, 1 to 4 in number of asci.
Yeast, which belongs to the group of bottom yeasts, forms on a “koji” extract a ring after 3 days. It fermented glucose, levulose, mannose, sucrose, maltose, but not galactose, lactose, or raffinose.
Zygosaccharomyces major, isolated by Takahashi and Yukawa from the maturing “Shoju” (Japanese soybean condiment), was met by Hall, James and Nelson in Barbados cane molasses, along with Zygosaccharomyces. Nussbaumeri Loghead and Heron.
Saccharomyces cerevisiae Hansen.
Round or oval cells. Limited development temperatures in beer must: minimum 13°, maximum 40°. Ascospores 1 to 4 in asci (sometimes 5), very cold and with a distinct membrane; diameter ranging from 2.5 to 6 mus. At 37° 5 and at 9°, the ascospores are not formed: they appear at 36-37° after about 24 hours; at 30° (optimum) after 20 hours, and at 11-12° after 10 days. Limiting temperatures for haze formation: 5° and 38°. The film [krausen?] appears at 33-34° after 9-18 days: at 6-7° after 2-3 months; and at 20-22° (optimum) after 7-10 days. At 20-34° the cells of the film have an elongated shape and a bizarre appearance; in the old films, we meet all kinds of cells, some very long, having the appearance of a mycelium. Yeast ferment glucose levulose, sucrose, maltose, but not lactose.  S. cerevisiae, found by Hansen in the breweries of London and Edinburgh, is very common in breweries and distilleries. Many yeasts, top or bottom, of which the systematic position is poorly known, are attached to this species. Particularly noteworthy are the Frohberg type and the Saaz type, the first with low attenuation and the second with average attenuation. These yeasts include various widely used breeds, especially those of the Frohberg group, in industrial fermentations.
S. cerevisiae, found by Hansen in the breweries of London and Edinburgh, is very common in breweries and distilleries. Many yeasts, top or bottom, of which the systematic position is poorly known, are attached to this species. Particularly noteworthy are the Frohberg type and the Saaz type, the first with low attenuation and the second with average attenuation. These yeasts include various widely used breeds, especially those of the Frohberg group, in industrial fermentations.  Saccharomyces Vordermannii Went and Prisen-Geerligs.
Saccharomyces Vordermannii Went and Prisen-Geerligs.
Round or piriform cells, measuring 6-7 x 5-5 mus, the youngest remaining long enough welded to the old ones. In old agar cultures there are also elongated, filiform cells surrounded by ordinary cells. Ascospores numbering four in each cell (2-3, according to N. Deerr.). Yeast does not form a film on the surface of sugary liquids, but only a ring in contact with the walls of the container.
It fermented glucose, levulose, sucrose, maltose and raffinose, but not lactose or dextrin. Fermentation, which is very fast, ceases in the presence of 9 to 10% alcohol.
S. Vordermanii was discovered by Went and Geerligs in the ragi, a starter used for fermentation of Batavia arrack. Some authors attach it to S. cerevisiae, the characters that distinguish the two species being of little importance.
S. Peck and N. Deerr studied isolated ferments of cane molasses samples from various rum producing countries (Cuba, Demerara, Java, Mauritius, Natal, Peru, Trinidad). They met only budding yeasts, except in the molasses of Peru, where there was exclusively a fission yeast. The differences they noted between the agents did not seem sufficiently clear to them to be considered specific. These authors look accordingly at the budding yeast isolated by them as varieties of Saccharomyces Vordermanni. From the physiological point of view, they develop slowly from 20 to 25° and very quickly to 32°: they ferment glucose, levulose, sucrose and maltose.
Pairault, who has examined rum yeasts from the French West Indies, considers that they, with the exception of Schizosaccharomyces, are related to beer yeast (S. cerevisiae).
“They are cordially round,” he writes, “sometimes oval, those of from vesou are generally smaller and rounder than those taken in tanks of molasses: the first are from 3 to 7 mus in length, the others from 7 to 9. Rum yeasts are fond of high temperatures, their activity is very low below 23°, their preferred temperature seems to be 35°, but some still stand valiantly at temperatures of 41-42 and even 43°. Many rum yeasts do not ferment maltose, out of 40 tried, 18 were in this case. In the same rhummeries, a few days apart, I have met yeasts that fermented maltose and others that did not. The others appear to be about as active, according to the tests made. Rum yeasts are generally held at the bottom of the vats, where they congregate in compact masses [flocculate]. Some yeasts are surface yeasts and form beautiful yellow skins above the vats, which are usually removed. These high yeasts are usually Schizosaccharomyces.”
Kayser has noted presence, in molasses received from French colonies (Martinique, Guadeloupe, Reunion), of both fission and budding, yeasts; bottom, top, and film. Surface Saccharomyces were generally not very active and had low fermentative power. Most of the yeasts examined had a heating time of 10 minutes at 55° and even one of them 2 minutes at 60°. The vast majority fermented levulose, glucose, sucrose, maltose, mannose, raffinose, galactose and inulin, but none attacked dextrin. Some top yeasts did not secrete sucrase and left sucrose intact. Alcoholic strength, quantity and quality of the acids (fixed or volatile) produced, optimum reaction of the medium, odor released during fermentation, appearance of giant colonies, etc., varied from one yeast to the other.
It is therefore probable that, besides yeasts of the cerevisiae group, there are other Saccharomyces in rhummerie musts, differing in particular in their action on various sugars and cultivation characteristics (formation of giant colonies). The identification and classification of these yeasts require further study.
Levure Logos.
Yeast of great industrial importance, but whose morphological characters have been little studied. It has elongated cells, resembling those of Saccharomyces pastorianus Hansen. Isolated by Van Laer and Denamur from yeasts employed at the Logos et Cie brewery in Rio de Janeiro, it seems to have originated from a spontaneous fermentation of cane juice. It is a bottom fermentation, slow yeast, producing little alcohol, but having a very high attenuation limit. It fermented glucose, levulose, sucrose, maltose, galactose, mannose, inulin and dextrin.  Saccharomyces ellipsoideus Hansen.
Saccharomyces ellipsoideus Hansen.
Elliptical or round cells, developing on beer must between 0.5 and 40-41°. Ascus generally ellipsoidal and small, containing 1 to 4 spores measuring 2 to 5 mus. At 4° and 32°5, the ascospores are not formed; they appear after 26 hours at 30.5-31°5; from 21 h to 25° (optimum) and 41 days to 10°5. A film is not formed at 5° or 38°; it is fully developed at 33-34° (optimum) after 8-12 days, and at 6-7° after 2-3 months. The cells of the film are often sausage-shaped. Yeast ferment glucose, sucrose and maltose. [Some of the degree signs in here get wacky and it may be worth consulting the original page. I can’t quite tell if he is talking about temperature, brix, or attenuation, and how he is denoting half measures.]  The ellipsoideus were discovered by Hansen on the surface of grapes. They play the leading role in the fermentation of wine, and many breeds are attached to it. Wine yeasts, especially those of Champagne, are quite often used in the manufacture of rum by pure culture; they are used especially in Cuba and sometimes in Martinique.
The ellipsoideus were discovered by Hansen on the surface of grapes. They play the leading role in the fermentation of wine, and many breeds are attached to it. Wine yeasts, especially those of Champagne, are quite often used in the manufacture of rum by pure culture; they are used especially in Cuba and sometimes in Martinique.
Saccharomyces Zopfii Artari.
Spherical or ellipsoidal cells, 3-6 mus of diameter. Maximum budding temperatures 33-44°, optimum 28-29°. Spherical ascospores, 1.5-3 mus of diameter, numbering 1-4 per cell (2 in general): maximum temperature of sporulation 32°. The vegetative cells can withstand a temperature of 66-67°, and even, according to Owen, 90° for 10 minutes. This yeast ferments glucose, levulose and sucrose, but have no action on maltose or lactose.
Isolated by Astari during the production of sugar in Saxony, Zopfii was found by Owen in the sugars of Cuba, where it is one of the main agents of deterioration of this product, as well as in “blaskstrap” molasses. Schweizer and Fischlin also found it in the musts of cherries and found that it produced high amounts of volatile acids and esters. The eau-de-vie obtained was of inferior quality and had a very pronounced fusel odor.
Like other Saccharomyces involved in the fermentation of cane molasses, the following species have also been reported:
Saccharomyces secundus Groenewege. This yeast, which is attached to the cerevisiae group, was found by Groenewege (1) in a Batavia distillery. It would play a relatively important role in fermentation.
(1) Arch. Suikerind. Ned, Indie XXIV, Med. N° 16, 1916.
Saccharomyces javanensis Groenewege. Same origin as the previous yeast; very secondary importance.
Saccharomyces formosensis Nakazawa (1). Isolated by Nakazawa, in Formosa, from a must of fermented cane molasses.
(1) J. Agr. Chem. Soc. Japan IX, 285, 1933.
Saccharomyces robustus Nakazawa and preciosus Nakazawa (2). Isolated from a cane juice must fermented in Manila, these yeasts, which have a high fermentative capacity, attack glucose, levulose, sucrose, galactose, mannose, raffinose and trehalose, but not maltose, inulin and dextrin. Optimum temperature: 33 and 33°5; Optimum pH 4.7-6.4 for the first species and 5.3-6.1 for the second species.
(2) J. Agr. Chem. Soc. Japan XII, 356, 1936.
Pichia californica Siefert.
Cells generally oval, measuring 4-8 mus long and 3-5 mus wide, enclosing a very refractive corpuscle. They form a delicate film, which falls to the bottom of the container when stirred. Limiting temperatures of budding, in a wine at 8°: 7-12° and 33°; optima 28-30°. In the must, the maximum temperature is 39°. Sporulation limit temperatures, 5-6° and 39-40°; optimum close to 34°. Spherical and refractive ascospores, measuring 2-3 mus of diameter, forming only in a 12% alcoholic solution.
Isolated by Siefert from a red wine from California, this yeast was found by Saito (3) in the fermentation of cane molasses musts on Bonin island (Japan) and would be the main agent of this fermentation.
(3) Z. Spiritusind. XXX, 565, 1908.
Willia (Torula) Van Overeem.
Isolated by De Kruyff from the “ragi” and the “tapé” of Java, this yeast plays an important role in the manufacture of Arrack from Batavia.
Torula spp.
These yeasts constitute a rather heterogeneous group, characterized by a generally spherical shape of the cells and absence of sporulation. They often ferment sugars, especially glucose and levulose. Many forms have been described by Hansen, Will, Pearce and Barker, Wehmer, etc …  Very common in molasses, Torulas are frequently involved in the spontaneous fermentation of sweet musts. Gifted with a great oxidizing power, they produce a lot of esters, whose quantity increases with the nitrogen richness and aeration. They are aerophilic, prefer amide nitrogen and have an optimum pH of 4.5, according to Kayser (1). [I suspect all they produce is ethyl acetate.]
Very common in molasses, Torulas are frequently involved in the spontaneous fermentation of sweet musts. Gifted with a great oxidizing power, they produce a lot of esters, whose quantity increases with the nitrogen richness and aeration. They are aerophilic, prefer amide nitrogen and have an optimum pH of 4.5, according to Kayser (1). [I suspect all they produce is ethyl acetate.]
(1) C. R. Acad. Agr. XI, 449, 1925.
Ashby in Jamaica has isolated a species that spontaneously ferments molasses, diluted or undiluted (90° Brix). It does not form a film, but only a ring. Incapable of inverting sucrose, it only weakly fermented cane juice; more, in molasses musts, it gives about 8% alcohol. It determines a very slow fermentation and produces considerable quantities of esters: up to 18% of the alcohol formed, after 24 days. [probably all ethyl acetate]
Browne has described, under the name Torula communis, a species he found in Cuban raw sugars, which fermented invert sugar, but not sucrose. It can develop in the most concentrated sugar solutions and plays an important role in the deterioration of raw sugars, especially between the age of 9 and 15 days after manufacture.
Monilia spp. — Odium spp. The genus Monilia includes mushrooms growing on beer must, in the form of a mycodermic film or, rarely, a simple ring.
Browne has encountered two species: Monilia nigra and M. fusca Browne, with black pigment, in raw sugar (which they cause deterioration) and cane molasses.  On sucrose agar, M. nigra‘s colonies first appear as star-like spots. They consist of hyphae arranged radially and giving rise to transparent cells. When colonies have reached a certain size (1-15 mm), the hyphae break up into clusters of black conidia. Yeast cells, elliptical in shape, can give rise to new hyphae or multiply by budding like yeasts. M. fusca differs from M. nigra in the greenish-brown coloring of conidia, the shorter length of hyphae, and less pronounced tendency to give yeast-cells.
On sucrose agar, M. nigra‘s colonies first appear as star-like spots. They consist of hyphae arranged radially and giving rise to transparent cells. When colonies have reached a certain size (1-15 mm), the hyphae break up into clusters of black conidia. Yeast cells, elliptical in shape, can give rise to new hyphae or multiply by budding like yeasts. M. fusca differs from M. nigra in the greenish-brown coloring of conidia, the shorter length of hyphae, and less pronounced tendency to give yeast-cells.
These two fungi grow well in raw sugar solutions, except the most concentrated ones, with light gas production and a fruity smell. They determine the inversion of sucrose.
*****
Went and Prinsen-Geerligs (2), in Java, isolated a yeast from “ragi”, which they called Monilia javanica and which, according to these authors, would be used in the fermentation of Batavia’s arrack. It appears in the form of rounded or piriform cells, sometimes irregular, forming, after 1-2 days, a film on the surface of sugary liquids. On agar, we obtain mycelial filaments, giving rise to yeast-shaped cells. M. javanica fermented glucose, levulose, sucrose, maltose and raffinose but not lactose or dextrin. It produces about 5% alcohol after about ten days. The resulting alcohol has a rather unpleasant taste.
(2) Verhand. Königl Akad. Weteneh, Amsterdam IV. N° 2. 1895
Peck and Deerr found in a cane molasses from Natal, a Monilia neighbor of the M. javanica. It presented the interesting property of giving during fermentation a fruity smell reminiscent of that of the best Jamaican rum. In pure culture on molasses must, it produced 7.5 esters per 100 of alcohol formed. The esters consisted mainly of ethyl acetate and butyrate. [that sounds promising!]
Various other species of Monilia have been observed to cause sugars to boil, giving alcohol. To mention in particular Monilia candida Bonorden and M. vini Osterwalder. Their fermentative power is usually low.
Finally, Arroyo, in Puerto Rico, used in fermentation of cane juice (see Chapter VI) an Oidium, O. suaveolens, found in the sap of a shade tree used in coffee plantations. This mushroom forms on the surface of musts a thin film. It has little action on sugars, but by attacking protein materials of the medium, it gives rise to significant amounts of organic acids and esters. The dominant smell of the bouquet is that of ripe apple.
2 thoughts on “Kervegant Chapter IV The Yeasts”