Follow along: IG @birectifier
[This was a ton of work!]
Kervegant Part 13 PDF [I still need to make a PDF of this complete chapter]
Pages 126-156
CHAPTER V
PREPARATION OF RHUMMERIE MUSTS
Must, or “grappe” (1), is obtained by mixing, in certain proportions, sweet raw materials (juice, syrup, molasses or scums) with vinasse and water so as to obtain a determined density. Acidity is usually adjusted by addition of a small amount of sulfuric acid. Sometimes an antiseptic and nutrient salts are added to facilitate yeast development. Proportions of various ingredients vary within wide limits, depending on the type of rum that one wants to obtain.
(1) The term “grappe” [cluster/bunch] is still widely used in the French West Indies from the beginnings of colonization.
Pretreatment of raw materials
Sweet raw materials used in the brewery have certain defects, which may need to be corrected in order to obtain a good fermentation and a better quality eau-de-vie.
Cane juice is very rich in microorganisms, some likely to cause lateral fermentations leading to production of aromatic principles unpleasant to taste. Molasses may contain, in addition to harmful ferments, excess mineral matter or organic acids which not only hinder development of alcoholic yeasts, but also adversely affect rum bouquet. Also, various authors recommend to pre-sterilize juices and molasses, and, in the case of the latter, to eliminate unwanted constituents. In preparation of yeasts for pure yeast fermentation, it is obviously essential to sterilize the must, at least partially.
Treatment of molasses.
Beet molasses, rich in organic acids harmful to yeast, were formerly treated by the de-nitration process (2). To molasses, diluted at 25°-28° Baumé, an amount of sulfuric acid was added (2 kgs., Acid at 60° B. p. 100 of molasses on average) sufficient to decompose organic salts, and it was boiled for a quarter of an hour in a steam-heated tank equipped with an air bubbler to facilitate removal of volatile acids. [wow, that is gnarly!]
(2) Today most plants have removed de-nitration, to use yeasts acclimated to the volatile acids of molasses or, more often to carry out fermentations in the presence of antiseptics (fluorides).
Except in the exceptional case of products having undergone a butyric or putrid fermentation during their storage, cane molasses, generally little charged with volatile fatty acids, do not require to be subjected to such an energetic treatment. Most often, they are used as such in the preparation of musts.
Barbet process. Barbet, however, recommends sterilization by means of apparatus similar to that used for denitration. He devised a continuous device, functioning as follows:
Molasses is diluted to 28-30° Baumé in 2 wooden or copper tanks, serving alternately, and added, if necessary, a small amount of sulfuric acid to remove fatty acids. It then passes inside the tubes of a tubular heat recuperator, heated by hot molasses from sterilization: a regulator makes it possible to feed the recuperator uniformly. At the outlet of the latter, molasses enters, almost boiling, in a sterilizer, constituted by a closed copper tank, made of a steam-bubbling heating system, a perforated tube allowing the injection of air in the liquid and a gas vent. If the vapors that emerge have a bad odor they are expelled into the atmosphere. In the opposite case, they are condensed in a tubular cooler and added to the must or, if one operates by the method of “repasse”, to “brouillis”.
On leaving the sterilizer, molasses flows into a dilution tank, to be brought to fermentation density, by mixing with hot water coming from the condensers or with hot vinasse coming out of the distillation column. A special device allows to adjust the proportions of these ingredients. The dilution tank is equipped with a steam bubbler, which allows wort to be warmed again after dilution and thus completes sterilization (1). The duration the must stays in the tank is 20 minutes. Finally, after heating the fresh molasses in the heat recuperator, the diluted molasses is brought to the temperature required for fermentation in a container, arranged so that no contamination of the must can during cooling.
(1) The temperature can be increased to 100° in the sterilizer and then in the dilution tank. However, with molasses feeds, which have an acidity generally greater than 2 gr per liter, practically sufficient sterilization is obtained by heating at 80° C.
In the case of small distilleries, the above device is simplified and reduced to a single tank, in which the molasses is diluted to 30° B., brought to boil and finally water or vinasse added to obtain the suitable density.
Arroyo process — Arroyo proposes to perform, in addition to a sterilization at 80° C., a heating (2) and an acidification of the molasses.
(2) Purification with lime was already recommended formerly. Unfortunately sulphate of lime tends to quickly incrust devices (trays, condensers, tubulars, etc.).
In an open cylindrical tank, equipped with a heating coil and mechanical stirrer, molasses is introduced, which is mixed with a certain quantity of milk of lime, determined in advance, so as to obtain a raising the pH of the raw material by 0.5. After addition of the milk of lime, the agitator is set in motion and hot water is introduced until the density of the mixture is 50-55 Brix. The temperature is then raised to 70-80° C., and maintained at this level for half an hour.
[I have a tiration method devised so I can know how much lime in grams/L it takes to raise the pH by this degree or often better to 6.2]
While continuing a vigorous stirring of the mass, at the end of this period of time, liquid is passed to a centrifugal separator (Alfa Laval centrifuge for example), to remove from it solids which have been precipitated or separated during treatment (3). Clear liquid is returned to a second tank similar to the first, but the coil is fed with cold water instead of steam. As soon as the coil is covered with liquid, cooling is begun and the agitator is set in motion. Once the temperature has fallen to 35-40° C, nutrient salts, if any, are added then the amount of sulfuric acid needed to obtain a pH between 5.0 and 5.6. The liquid passes a second time, for clarification, to the centrifuge, and is sent to “composition”.
(3) One can also use for this purpose, either a pressure filter or decantation, But the most expeditious process is that of centrifugation. [Arroyo later demonstrated decanting. We’ve widely explored centrifugation and even identified many of its drawbacks.]
This molasses pretreatment not only destroys microbial flora, allowing only heat-resistant spores to survive, but also determines chemical modifications of the medium, which facilitate fermentation progress and favors yeast production of aromatic principles improving rum aromatics (mainly rum oil).
Lime neutralises fatty acids present in molasses, thus preventing their volatilization during subsequent heating. These acids are then released, after the addition of sulfuric acid, and intervene in the constitution of the bouquet of the brandy. It continues, under the combined action of pH and heat variations, abundant precipitation of organic impurities (gums, etc.) and minerals which increases saccharine richness and decreases liquid viscosity.
Here are some analyzes, from Arroyo, which show modifications brought to the material by the treatment (figures related to the primitive molasses):

Treatment eliminated 30 to 40% of the mineral substances and gums, with consequent increase of the sugar content of 3 to 5% and a decrease of the Brix density of 4 to 7%.
Benefits of molasses purification are not limited to fermentation. Wines sent to distillation being clear, one avoids clogging of the columns, reduces vapor expenses and improves output of the apparatuses. The bottom of the tank is composed almost exclusively of yeast. Recovery of the latter, in the form of fodder yeast or food, is greatly facilitated. Finally, pre-treatment increases concentration of potassium salts in the vinasse and makes their recovery more economical by concentration and incineration. [Not sure about this last potash point. My understanding is that it is a challenge to dispose of regardless.]
Cane juice.
Arroyo advises treating cane juice as molasses, by defecation with lime. Vesou is brought to 80° C, milk of lime is added to have a pH of 7, then sulfuric acid is added to reduce the ionic concentration to pH 5.8. It is then clarified by filtration or centrifugation. [I’ve always wondered if there was a solar solution to achieving this.]
This treatment regulates and accelerates vesou fermentation, destroying many microorganisms that are in the liquid, while improving rum bouquet. In particular, it increases production of rum oil by certain breeds of yeast. Liming, if not done with great caution, may, however, have deleterious effects, an excess of lime liberating organic bases and bodies of the group of alkaloids, which can pass into the distillate and communicate to the rum an unpleasant taste.
[I have solved this liming precison problem by developing a titration method that tell you exactly how much lime to add to hit a precise pH.]
Arroyo gives the following results, obtained using the same raw cane juice (1), (2) heated to 80°, (3) heated and defecated with lime as indicated above. During fermentation and distillation, conditions were kept as similar as possible for the 3 samples.

As early as 1895, Greg drew attention to the role that liming played in the production of rum aroma, which he attributed to a particular essential oil. During experiments in Jamaica, he noted:
(1) that un-limed cane juice and rums derived therefrom did not contain rum oil;
(2) that vesous defecated with lime and having fermented under the action of certain yeasts (No. 18 in particular) contained rum oil;
(3) that industrially produced rums, made from a mixture of cane juice, molasses and scums of defecation [skimmings], also contained, in very variable proportions, the same essential oil.
The author accordingly admitted that this aromatic matter resulted from the action of certain yeasts, made possible by the lime. Excessive liming releasing organic bases from the pyridine group also spoiled rum quality. These conclusions have been confirmed in recent years by Arroyo, who has specified, depending on the pH, the optimal amounts of lime to use. [Circular 106]
In the French West Indies, where vesou rum has been produced for more than half a century, distillers have also become aware of the influence of defecation on the quality of eau-de-vie. They have known for a long time that heating vesou gives a bouquet to the rum that is more full-bodied and more persistent, aging more quickly. Liming, followed by a concentration of the liquid, accentuates these qualities, at the same time that it makes the special aroma of raw juice, called “vesouté”, disappear.
However, in Martinique and Guadeloupe, it is the manufacture of rum of raw vesou that prevailed over that of rum of cooked vesou and rum of syrup. The first product is, in fact, easier to obtain by spontaneous fermentation, and its bouquet appeals more to Creole consumers. However, from an export point of view, the other two types have indisputable advantages.
In Jamaica, on the other hand, cane juice used in the composition of musts is very often defecated with lime. This operation is considered essential for production of quality rum.
Must composition
Musts of vesou and syrup.
The operation which consists in mixing raw materials entering the must is known, in the French West Indies, under the name of “composition”. [Arroyo used the term batición sort of like cake batter.]
Vesou musts are often composed by simply diluting cane juice with water, so as to have a density varying between 1.035 and 1.065 and a sugar content of 8 to 14%. In Martinique, the density is normally between 1.035 and 1.045 (at the temperature of the observation), while in Madagascar it reaches 1.060-1.065.
When it is desired to obtain a more full-bodied product, more particularly suitable for export, water is partially replaced by vinasse. In this case, the composition usually varies between the following limits:

Pairault indicated at the beginning of the century as the average composition of vesou musts in the French West Indies:

Syrup musts are sometimes prepared simply by diluting syrup with water, so as to have a density of 1.043 – 1.050. More often, however, a high proportion of vinasse (60 to 70%) is added, drum syrup rums being generally intended for export and having to be consequently more full-bodied. Hereinafter the composition adopted for musts of a well-known brand of rum from Martinique: [I got drum from their use of “batterie” which I think implies storage.]

Fermentation of the must above, abandoned for spontaneous seeding, is rather slow (5-6 days); rum obtained has a coefficient of impurities close to 400. [that really looks like it translates to abandoned!]
In Haiti, where the main raw material used in the production of rum is drum syrup, proportions would be on average, according to Pairault:

Fermentation lasts about eight days, sometimes less, often more, [pombe yeasts…]
According to E. Baker (in Litteris [correspondence]), the must is currently obtained by mixing 35-38° Baumé syrup with water in the ratio of 1 to 4 or 6, so as to obtain a liquid of density varying between 8° and 10° Baumé. In general, vinasse is added to the composition in a proportion of 1/5 to 1/3. Some distillers, very rarely indeed, use Ammonium sulphate and sulfuric acid, at the respective doses of 0.5 and 1 p. 1,000. Fermentation lasts from 3 to 8 days, more often from 6 to 8 days. This variability is explained by the following reasons: no seeding is done at the time of fermentation; the foot of the vats [pieds de cuve] are only exceptionally used; temperature of the fermentation rooms varies considerably from one season to another; finally the density of the musts is not regularly followed. [They may likely be riding the line between a Pombe ferment or not.]
Manufacture of syrup rums was known in the Antilles as early as the 18th century. Ducœurjoly writes about it:
“We express the cane juice, crushing them in the mill, in the usual way: one cooks one third of this juice, or vesou, until the consistency of syrup; we take the other two thirds, which is boiled for about an hour, and until it has rejected all the coarse skimmings that come to the surface of the chauctère. This latter liquor is used instead and in the same way as the scums, and the first to hold place of syrup … ” [SOS this may not be translated the best, especially the last line.]
As another way of preparing grappes with cane juice, the same author reports: “The vesou is cooked, in the good foaming, until the consistency of light syrup, the froths that have been drawn serve instead of the fact that boilers are extracted, when sugar is made. In the composition of grappes, this syrup and foam are twice as much as if it were sugar syrup and ordinary scums. The rum that is distilled is very good, and it is called, in Barbados, where he manufactures a lot, spirit of rum”.
[This old language is very tricky to translate. Context only gets you so far. There are observations to be made about the skimmings and I believe there are positive types from cane wax and other types that may just be dirt.]
Molasses musts.
Molasses must compositions present great variations, according as one wants to have a more or less aromatic product.
Often, it is enough to dilute molasses with water. This is how they operate in particular in English Guiana, where they add the amount of water needed to have a density of 1.060-1.063 to the final molasses. The proportion is 15-16 volumes of molasses per 100 volumes of water and the total sugar content of about 9%. ###
In the United States, “blackstrap” molasses musts for alcohol manufacture have a density a little higher: 1.065-1.075 in general. In some cases, however, it is 1.080. The normal dilution rate is 1 volume of molasses per 5 volumes of water. ###
In the French colonies (Martinique, Guadeloupe, Reunion), musts are also sometimes composed with only molasses and water. More frequently, however, a certain amount of vinasse is employed. This is 10 to 30% generally in Reunion and La Guadeloupe.
In Martinique, vinasse amounts to 50-80%. In some factories, it even reaches 90%. It is then no longer used with water, except in very small quantities to bring back to the initial degree the density of the vinasse, which is concentrated as the production season progresses. The proportion of molasses (by volume) is usually 10%; it rarely reaches 15%. As for the density of the must, 1.040-1.050 most often, it rises to 1.075-1.080, when one wants to get a full-bodied rum. The sugar content is usually between 6 and 8%. Exceptionally for the manufacture of grand arôme rum, the must, composed only with molasses and vinasse is very acid (15 gr per liter) and high density (1.090), reaches up to 1.115: fermentation is in this very slow (9-10 days). In the old industrial distilleries of Saint-Pierre, the most used proportions were: ###

For the manufacture of certain types of rum, mixed musts of vesou and molasses are prepared. According to Suzuki, we would obtain the best results from the point of view of fermentation and alcohol yield, by using a mixture of 60% of vesou at 15° Brix and 40% of molasses at 20° Brix, added with 50 gr. Ammonium sulphate per hectolitre of must.
Molasses and scum musts. [skimmings]
In the beginnings of the rhum industry, musts were generally composed of a mixture of molasses, defecation foam and vinasse.
Ducœurjoly (1802) indicates, for the composition of grappes, various formulas, according to the nature and quality raw materials available. He advises, for example, for the beginning of the season, when one does not yet have syrup (molasses), and per tank of 300 gallons:

When you start having some molasses, Ducœurjoly reports the following combinations:

For the time of the harvest:

To continue to make rum after the end of the sugar manufacture, when we no longer have scums:

Finally for grappes without scums or dunder [vidanges]:

“24 h. after and when you have brewed and skimmed well, add syrup”
Soleau (1) wrote in 1835 about rum making in English Guyana:
(1) Ann Marit. Col. 1835, t. 2, 40.
“In general, there is little molasses distilled, the sale being more advantageous and not giving a rum as perfect as that which is obtained with scums. Proportions of the different parts that make up the grappe vary with the molasses amount that we have, or that we want to distill. Here are the proportions of a grappe: it contains 30 gallons of scum, 30 of water, 30 of dunder, 10 of molasses. It is calculated that 6 parts of skimmings correspond smoothly to a part of molasses. Now, if we vary the respective quantities of skimmings and molasses, we manage to have, by the reduction and the number we give to the foam, 15% of douceur [translates as gift payment, so extra?] and to complete the 100 parts in quantity by water and dunder”.
Porter indicates as proportions frequently used in the beginnings of the last century in the West Indies:

The amount of sugar was about 15%.
In Jamaica, preference was given to:

Average density was 1.050 and sugar content was 12%. “In some distilleries, Porter writes, the sugar content is 14-15%, which does not seem to be exceeded, because the formation of too much alcohol prevents the fermentation from ending and the sugar to be completely transformed”.
Wray recommends a similar mixture consisting of 10% molasses, 20% skimmings, 50% vinasse and 20% water.
At present, we continue to use scums more than in Jamaica. In this country, ingredient proportions used vary from one distillery to another, according to the rum type that one wants to obtain (common clean, medium rum or german rum). Usually musts are formed by 10% molasses (70 to 85° Brix), 20 to 40% skimmings and 10 to 20% water. ###
Density varies from 16 to 25° Brix, musts for grand arôme rum being generally thicker than the others. Sugar content varies between 8 and 15%; in some rare cases, it may exceed 16%. Acidity is relatively very high: from 10-15 gr per liter (as sulfuric acid) on average, it sometimes rises to 20 gr and rarely drops below 8 gr. This acidity, due to the use of scums and acidic vinasse, is constituted, in the proportion of 70 to 90%, by fixed organic acids. [If we want to convert the acid as sulfuric to acid as acetic, add 20%] ###
Fermentation time, from 4-6 days for light rums, reaches 2-3 weeks in the case of grand arôme rums.
In distilleries not annexed to sugar factories, skimmings are replaced by cane juice, which is limed and often left to itself for a few days in a juice tank before being used. [This may be what distilleries are presently practicing.]
For manufacture of german rum, a certain quantity of aromatic liquids, acid (acid) and l’arôme (flavor) obtained by subjecting the cane juice to a special treatment, are added to the must (see Chapter II). Arroyo gives the following example of grand arôme rum composition:

[Its funny he quotes Arroyo who never made these rums nor ever really commented on skimmings or dunder. Arroyo got the data from old Jamaica literature.]
Practice of composition.
In the past, must composition was often done in several stages. “In the Leeward Islands,” Porter writes, “vinasse and water are sometimes mixed together in equal proportions. When these ingredients are well mixed and the temperature is appropriately regulated, fermentation is sufficiently advanced after 24 hours to allow the addition of the molasses, which is introduced in the proportion of 3 gallons per 100 gallons of liquid. After 1 – 2 days, when everything is well fermented, pour the same amount of molasses again.” The author advises, however, to use molasses in one go, after the beginning of the fermentation, successive additions tending to stop fermentation and lengthen the duration.
Wray indicates the following process for composing a 1,000-gallon tank. “First of all, 200 gallons of well-clarified skimmings are mixed in, 50 gallons of molasses and 100 gallons of clear vinasse are mixed together, then the fermentation is allowed to settle in. This is done very quickly. 50 more gallons of molasses, 200 gallons of water are added, and the mixture is left standing for an hour for the fermentation to take place, at which point 400 gallons of vinasse are poured into the tank and mix thoroughly with the mass”.
Even today, in certain regions where the manufacturing processes have remained primitive, the must is prepared in stages. Thus Prinsen-Geerligs reports the following operation mode observed by him in a distillery on the peninsula of Malacca [Malaysia]. Molasses is diluted with water to a density of 15° Baumé and poured into large vats which are half filled. After 3 days, when the fermentation is almost complete, the tanks are filled to the top and distilled 3 days later. “If the tanks had been filled from the beginning,” writes the author, “the rise in temperature and the formation of acetic acid would have been such that the yield of rum would have diminished a great deal.”
De Sornay, in Mauritius, indicates a similar procedure, “Some distillers, instead of diluting the molasses with water at one time, dilute first at 16 or 17° Baumé, seeding [ensemencent? add yeasts?] and it is only when the ferment is well developed (7 or 8 hours later), that they continue the addition of cold water to arrive at 10° Baumé”.
In Haiti, one also operates on the composition several times [very interesting phrasing!]. Quantities of syrup and water required are poured into vats and, after fermentation has begun, the vinasse is added. Some put the first part of the syrup and water to start, then the second half of the syrup, then the vinasse (Pairault).
On the other hand, in rum producing countries where manufacturing methods have been modernized, must composition is generally done in one go. It should be pointed out, however, that Arroyo has recently recommended a method of fermentation with a thick must in which the addition of molasses is done in several stages.
Depending on the importance of the installation, the composition [batición!] is carried out either in fermentation tanks themselves, or in a special vat (which should preferably be copper and cylindrical) or a pit dug in the ground. The first way of doing this has the disadvantage, in the case where one practices seeding, to oblige adding yeast to the must, whereas it is better to pour the must on the starter. [This is a big Arroyo idea, must should be added to yeast, not yeast to must and he describes the reason in Circular 106.]
In the French West Indies, if small agricultural distilleries usually mix in the fermentation tanks, industrial rhummeries most often use a masonry pit for this purpose, embedded in the ground and coated with a cement layer. The capacity of this varies between 5,000 and 10,000 liters.
Mixing can be carried out as follows. First we get molasses, whose volume is measured by passing through a special tray under load, or using a graduated rule placed in the pit. Vinasse, which comes from the boiler of the distillation apparatus, is then directly poured without being cooled or, more often, after passing through a tubular cooler which lowers the temperature to 30°. Finally, complete with water. The volumes of vinasse and water are generally measured by means of a graduated rule placed in the composition pit. In other cases, vinasse is first poured, which is brought to the desired density by addition of water, then the molasses and finally the water.
Mixing is carried out in small installations by means of a board of rectangular or rounded shape pierced with holes, placed at the end of a long handle. In larger plants a mechanical stirrer or an air injector is used. The latter is not advisable when working with sterilized musts, because, unless previously filtered or disinfected by passage through an antiseptic liquid, the injected air introduces foreign micro organisms into the liquid. As filter maintenance is a rather delicate operation, it is preferable to use a mechanical stirrer to perform the stirring.
Most often, we calculate the proportion of materials to be used to obtain a given density, as follows:
The difference between the final density and the densities of the two component materials is differentiated; the remains, placed inversely, indicate the proportions to be adopted. Or, for example, to mix a 1.400 density molasses with water, so as to have a wort at 1.050 density. The calculation is as follows:
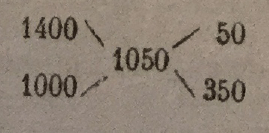
The proportion will be 50 liters molasses for 350 liters of water. This method of calculation gives only approximate results, but which nevertheless suffice in practical conditions, where one does not try to obtain a very rigorous density.
Vats and composition pit must be kept perfectly clean. It will be good, at the end of the day, to wash them with hot water or with exhaust steam, then with an antiseptic solution. The next morning, wash again with warm water. Carefully avoid leaving must bottoms.
Saccharine richness and must density.
In order for the alcoholic fermentation to take place under good conditions, sugar concentration of the must should be maintained within certain limits.
A too low density favors development of certain parasitic ferments and hinders that of the yeast. It is not advisable to go down, although it is sometimes done in a vesou distillery, below 1.040 (at 15°C), a density which corresponds to a sugar content of 9-10% in the case cane juice or syrup and 7-8% in ordinary molasses musts. ### [I think that is 10 brix and a potential alcohol of 5.2%. It is less in molasses because there is so much unfermentable material. This is important to consider if you are doing incrementally fed batch fermentations.]
On the other hand, it is economically advantageous to work with as concentrated liquids as possible. This makes it possible to reduce the necessary vat room capacity (and consequently first establishment costs), fuel expenses for distillation column and pump operation, as well as volume of vinasse obtained.
Factors determining concentration.
The factors that intervene to limit the concentration are: duration of the fermentation, action of non-sugar constituents, tolerance of the different yeast raceswith regard to cellular metabolism products, finally temperatures reached during fermentation.
At a certain saccharin concentration, close to 15%, transformation of sugar slows down rapidly, because of the inhibitory action exerted by alcohol on the yeast. In the manufacture of industrial alcohol it is not practical, for reasons of cost, to extend fermentation duration beyond 48 hours. Consequently, the concentration limit of the musts in sugar is located at about 15%.
It is not the same in the rhummerie, where costs do not need to be as tight. Prolongation of the fermentation is even advantageous in the case of grand arôme rums, because it is accompanied by a more important formation of aromatic secondary products (esters, acids). A short fermentation is indicated, however, for obtaining light rums. The type of eau-de-vie that one wishes to obtain will therefore be the main factor determining saccharine richness and must density.
The nature and proportion of non-sugar elements are also important. Cane juices as well as syrups are usually relatively poor in nutrients, especially if they have been deprived by the defecation of a certain amount of nitrogenous and phosphatic materials. This defect, increasing with dilution, to allow normal fermentation nutrition makes it necessary to raise the saccharine concentration around the maximum tolerated by yeast for the complete transformation of the sugar into alcohol in a normal lapse of time.
[This is worth pondering. It means you may want to front load nutrition for incrementally fed batches that start a low brix.]
In the case of molasses, on the contrary, especially when they have been used up, a high proportion of certain mineral substances may considerably hinder or even stop the fermentation, if the dilution ratio of the raw material is close to the maximum concentration of sugar. Vinasse intervenes in the same direction, the inhibitory action of salts being in this case increased by that of certain organic acids. The depressive effect of non-sugar constituents is especially pronounced towards the end of the fermentation, when only small quantities of sugar remain in the musts. The presence of residual sugars in wines is often much less the infermentable character of the latter than an unfavorable balance between non-sugar and sugars, the exhaustion of the nutrient medium and the production of toxins by the yeast.
[I think there may be a translation logic issue at the end of this paragraph.]
Also, the saccharine richness of molasses musts, especially when the water is partly or wholly replaced by vinasse, is usually lower than that of vesou musts or syrup: 9 – 12% instead of 10 – 14%. Sometimes, it falls to 7-8%, although the density of the must is very high (1.100 and more for the must of grand arôme rum in Martinique). [1.100 is 23.8 brix] ###
[We will have to look out later for for numbers on grand arôme dunder density.]
In the case of rhummerie ferments, other products of cell metabolism concurrently intervening with alcohol, to disturb the yeast: esters, higher alcohols and especially acids. The antiseptic power of these, practically negligible in the production of industrial alcohol, is all the more accentuated when one aims to obtain a more robust eau-de-vie, that is to say, richer in aromatic principles.
Behavior of different yeast races in musts with high saccharin concentration is also very variable. Here are some results obtained by Arroyo, in Puerto Rico, by fermenting with pure yeast musts with various sugar contents:
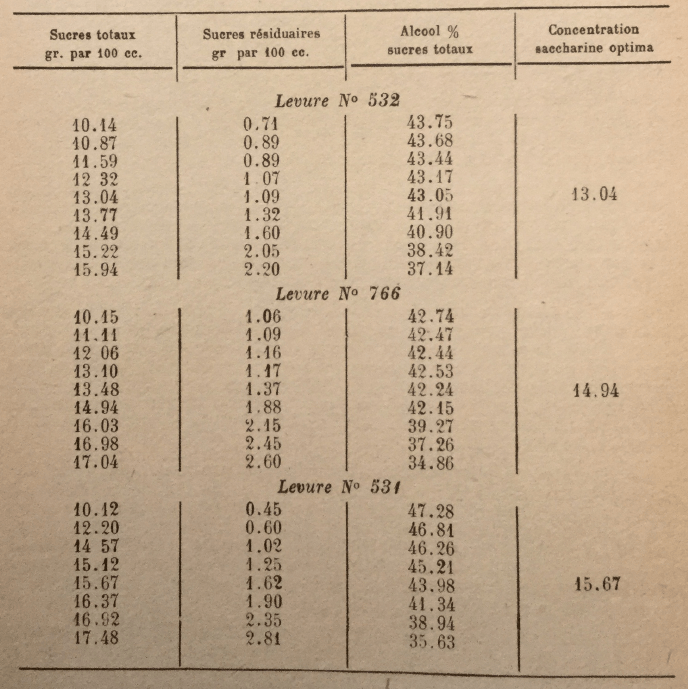
The above results were obtained at the Laboratory under optimal conditions of fermentation (temperature, acidity, etc.). In industrial manufacturing, where optimal conditions are rarely achieved, sugar concentrations must necessarily be lower.
It is generally accepted that the sugar content of musts for light or medium rums must be between 9 and 11%. In some cases, however, the rate is substantially increased, and up to 16%. This is particularly the case in the manufacture of the German Rum of Jamaica, or fission yeasts, particularly resistant to alcohol and acids. But then, fermentation duration is abnormally prolonged and alcohol yield often mediocre (1).
(1) However, by the process of yeast recovery, the wealth of alcohol can be pushed to 12-13% and even 14%, following observations made in France in 1944, in the work of sugar skimmings (Mariller). [Nothing by Mariller from 1944 appears in the bibliography, but he is a very important author and pen pal of Kervegant.]
As we have previously reported, temperature rise increases the antiseptic action of alcohol. While many yeasts can be acclimated to high alcohol concentrations, few are able to withstand the combined effects of high concentrations and high temperatures (Owen). In the tropical distillery where, as a result of cooling water temperature, it is generally not possible to go below 30° and where the temperature is sometimes raised to 38-40°, it would nevertheless be possible in many cases, to obtain much better fermentations, by acclimatizing ordinary yeasts to these unfavorable conditions (Owen, Chaturvedi). This acclimation is usually accompanied by a slowdown in the speed of fermentation.
The state of yeast development intervenes finally in resistance to the temperature-alcohol complex. When the musts are pitched with an insufficiently ripe yeast footing, containing a large proportion of young cells, it is not rare that the fermentation stops prematurely, before complete exhaustion of the sugars. [I think the logic of this may be backwards. I think you want young and vigorous cells with strong cell walls.]
Increase in the concentration of musts.
In addition to yeast selection and acclimation, various processes have been recommended to allow fermentation of musts with high saccharin concentration. These processes have been studied for the production of industrial alcohol. Tests should be done to specify the action they can have on the formation of principles of the bouquet and on their value in the rhummerie.
Delbruck observed that the addition of rye grains to concentrated sugar solutions improved fermentation. He attributed this effect to the mechanical action exerted by the grains, which facilitates the elimination of the carbon dioxide produced. [I think this is accurately translated as rye: “grains de seigle”. I’ve heard of this done with bagacillo which is pulverized bagasse to restart a stuck fermentation, but I’ve never encountered an explanation of the mechanics.]
Owen found that charcoal and cane bagasse enjoyed the same property. The use of activated carbon at a dose of 200 gr. per 1,000 liters, allows raising the initial density of the must to 30° Brix and more, while the maximum concentration generally accepted for the manufacture of alcohol industry is only 20° Brix. In his research, Owen observed that not only was the alcoholic yield of musts at 35° Brix with added carbon as high as that of ordinary musts at 18-20º Brix, but that acceleration of fermentation was such that the time required for the exhaustion of sweeteners was about the same in both cases. [I wonder if these days ultrasonic treatment could de-gas the beer.]
Bagasse also has a very clear accelerating action on the fermentation of molasses musts, especially when they have a high density. This action is however less pronounced than in the case of carbon. It is even larger if the amount of bagasse is higher: however, beyond 25 gr per liter, there would be no appreciable increase in stimulating action.
The yeast recovery fermentation method (see Supra) makes it possible to significantly raise must density. In the Hildebrandt and Erb process, alcohol yield is higher when the sugar content is higher, as shown, for example, by the following figures given by Owen (1):
(1) Sugar XXXVI, NO 3, 26, 1942.

Similar results were obtained by Owen, when introducing autolyzed yeasts into the musts from the treatment of bottoms. [This may be a way to increase yeast nutrition?]
Finally, Arroyo, by purifying molasses so as to eliminate substances (gums, excess mineral matter) and undesirable microorganisms, was able to obtain excellent fermentations with musts containing 18 to 22% sugar and giving wines at 10%-11% alcohol. During tests carried out, fermentation duration usually varied between 24 and 48 hours and alcohol yield was between 90 and 100% of the Pasteur coefficient.
In the Arroyo thick-must process (2), molasses is diluted with water, so as to have a density of 55-63° Brix, and then concentrated sulfuric acid is added in a quantity sufficient to lower the pH by 0.5, and, if applicable, nutrients (Am sulfate, lime superphosphate). The thick must thus obtained is carried in a tubular heater at a temperature of 80° C. During this time, mineral or organic impurities precipitate, microorganisms are destroyed by autolysis and sucrose undergoes some inversion. The clarified liquid is then decanted and sent to the fermentaton, while the deposit is taken in a special tray with two or three times its volume of water, to recover the sugars contained therein. After re-settling, clear supernatant water is used to prepare the normal must and the residue is discarded.
[Keep in mind this is for Arroyo’s treatment for ethanol, not fine rums. There is another version that uses lime instead of sulfuric acid.]
(2) U S. Patent 420 898, 28 Nov. 1942. By thick must, one hears a very concentrated mash. In France, this expression is reserved for liquids containing suspended solids (musts of grains, potatoes, etc …)
Clarified thick must from the settling tank is cooled in a temperature exchanger at 40° or below, before being sent to the fermentation tanks, where it is diluted with water.
The quantity of water necessary to carry out the desired dilution is first introduced into the tank, which is closed and equipped with a mechanical stirrer, then, progressively and continuously stirring, 50% of the thick must is introduced, so as to obtain a normal must having a density of 18 to 24° Brix. We adjust the acidity so that the pH is about 5.0 (variable depending on the yeast race used), and finally we add a starter or leaven, equivalent to 10 or 15% of the total volume of the must. After 12 hours, when the Brix dropped from 55 to 63%, 30% thick must is again introduced with gentle stirring and, after another six hours, the remaining 20%. The density after mixing must be a little lower than the initial density: if it is 22° Brix for example, the density following the first molasses intake will be 20° Brix and following the second intake of 18° Brix approx. ### [flagged for the high volume of the starter]
During fermentation, temperature is maintained between 28 and 30° C, by continuously passing the wort in an external refrigerant, and pH to 5.0 -5.2, by addition of lime or, better, of 10% ammonia water. To reduce the amount of foam that forms at the time of thick must additions, a small quantity of anti-foam product (“Turkey red oil” for example, with 1 liter of oil per 16,000 liters must). The fermentation is usually completed 15 to 30 hours after the last introduction of thick must.
This process has important advantages. The must is freed, by preliminary purification, not only of foreign ferments, but also of a large part of the mineral and organic impurities present in the molasses which counteract fermentation. Fermentation efficiency is substantially increased, and column fouling reduced. As must density is higher and wine alcoholic richness raised, distillery capacity is increased by 35 to 50%, the expenditure of manpower and fuel diminished.
We reproduce some data relating to fermentations carried out by this process in a distillery of Puerto Rico (1).
(1) Sugar XXXVIII No 2, 18, 1943

Acidification.
Must acidity deeply influences the process of alcoholic fermentation. Concentration of Hydrogen ions acts both on fermentation development and on the nature of the products formed. Insufficient acidity promotes the multiplication of bacteria, and causes yeast formation of a relatively large amount of organic acids at the expense of alcohol production. An excess of acidity paralyzes the yeast. Proper acidification of musts is therefore one of the most important points in the production of industrial alcohol and spirits.
This acidification is performed in the rhummerie by the addition of sulfuric acid or acidic vinasse. In the grain distillery, it is sometimes acidified, but more and more rarely, by subjecting the must to a preliminary fermentation by lactic acid bacteria. When sulfuric acid is added to a solution of molasses, it is first applied to the alkalis, which are neutralized. Then it releases volatile organic acids. When the addition of acid used is quite high, fixed organic acids are in turn displaced and the excess of sulfuric acid remains, if necessary, in the free state. Complex nitrogenous materials are more or less degraded, according to the dose of acid, and transformed into more assimilable substances for the yeast. Finally, sulfuric acid begins the reversal of sucrose into glucose and levulose. This hydrolyzing action is however quite weak in practical conditions: it takes some importance from 1 gram sulfuric acid per liter.
Practice of acidification in the rhummerie.
The addition of sulfuric acid to molasses and vesou musts is a practice that has been in use for quite some time.
Pairault reports that at the end of the last century a large number of distillers in the French West Indies and English Guiana added small quantities of sulfuric acid to their composition in order to obtain purer fermentations. At present, the use of sulfuric acid is widespread, in the manufacture of ordinary rums. On the other hand, when one wants to have a grand arôme rum, one carefully avoids using it, the degree of acidity necessary for the yeast being obtained by the addition of vinasses or scums.
It has been proposed, on various occasions, to substitute for H2SO4, various other mineral or organic acids: hydrochloric acid (Mohlant), hydrofluoric acid (Effront), phosphoric acid (Collette and Boidin), lactic acid, etc. The use of these has not developed. However, hydrochloric acid is sometimes used in sugar beet molasses distilleries, to avoid encrustation of the columns and vinasse evaporators (formation of soluble lime chloride).
The quantities of sulfuric acid added to the must are quite variable according to country, type of rum that one wants to obtain, quantity and acidity of vinasse entering the composition, etc…
In English Guiana, 1 liter is usually used of acid for 1,000 liters of must. It is about the same in the United States. In the Magne process, which is frequently applied in the latter country, fermentation tanks receive 0.80 to 1 liter of acid per 1,000 liters must, intermediate tanks 1.20 liter for 1,000 liter and leavening appliances an even higher dose. [This is all from before the pH era.]
Martinique has used in manufacture of ordinary molasses rum 0.1 to 0.4 liters and in vesou rum 0.2 to 0.5 liters H2SO4, for 1,000 liters of must. The acidity, provided by the vinasse, is 1.4-1.6 grams per liter for cane juice musts and 3-4 grams for those of molasses. Exceptionally, it reaches up to 15 grams in the case of grand arôme rum. ###
In Guadeloupe, a small amount of sulfuric acid is also usually added to molasses musts, so as to have an acidity of 2 – 3 grams per liter, while in Madagascar and Reunion, acid is rarely used. In the latter two countries, the initial acidity of musts, often consisting of a simple mixture of molasses and water, is low: 1.5 to 1.9 grams per liter.
In Jamaica, sulfuric acid is never used. As the composition of high proportions of skimmings, vinasse and fermented juice (acid, flavor) are very acidic, the acidity of musts is dependently high: 10 to 20 gr per liter. ###
As regards the influence of vinasse on distillate composition, we can notably mention the observations of Kayser, who has noticed a significant increase in the rate of volatile acids. This author obtained, among others, the following results, with two musts with 12% molasses by volume. The first prepared with water, the second replacing half of the water by an equal volume of vinasse:

Adjusting the acidity.
Most often, in the rhummery, sulfuric acid addition is made empirically without taking into account compositional variations of the raw materials and the special requirements of the ferments employed. It is only in recent years that the conditions for rational acidification of the nutrients have been specified by means of pH control.
At the beginning of the century, Pairault warned Rhummeries against the use of an excessive quantity of sulfuric acid, which paralyzes the action of sucrase at relatively low doses. He indicated as the maximum quantity, that of half a liter of H2SO4, for 1,000 liters of must which it is most often good to reduce.
Humboldt (1) recommends adding only 1 liter of acid for 10,000 liters of cane molasses, and Arnstein (2) a sufficient dose to obtain an acidity corresponding to 1.5-2 cc of normal soda per 100 cc. Next, according to Effront and Prescott, the best practice for cane molasses distilleries would be to achieve an acidity of 1 to 2.5 grams per liter, whereas, according to Williams, molasses must should have a free sulfuric acidity of 0.1%.
(1) Louisiana Plant. LXVIII, 206, 1922.
(2) Louisiana Plant. LXVIII, 126, 1922.
Freeland insisted that the amount of sulfuric acid used must be a function of the quality of the molasses and the type of fermentation. A mediocre molasses requires a higher proportion of acid than another of easily fermenting good quality. In pure fermentation, much less acid is required than in spontaneous fermentation. “It is generally admitted,” he says, “that the appropriate acidity of the must is between 0.15 and 0.20%, evaluated as sulfuric acid, but this dose is often exceeded and may be as high as 3%, if the molasses contains a lot of free acids. Usually 0.75 to 1 liter of sulfuric acid is added per 1,000 liters of cane molasses.
Owen and Bond found that the dose of sulfuric acid corresponding to the maximum alcoholic yield varied according to the yeast used and molasses composition. They obtained the following results, during tests carried out with blackstrap molasses musts (at 16-17° Brix):

These results show that the optimum dose of sulfuric acid depends on the race of yeast. It is, with yeast N° 83 for example, of 1.50 cc. for Louisiana molasses and 1.25 cc. for that of Cuba. In the case of Magné yeast, the maximum alcohol yield is given by an acidification of 1.25 cc. for both types of molasses. The tolerance of the various breeds with regard to variations of the acidity is greater or less: the difference between the maximum and minimum alcoholic yields corresponding to the various acidity levels is highest for the yeast N° 74 and lower for Magné yeast. The amounts of acid required to obtain the maximum alcohol yield varied more in the case of Louisiana molasses (1.0 to 1.5 cc, depending on the yeast races) than in the case of Cuban molasses (1.25 cc). This seems to be due to variations in the “buffer power” of molasses. [Magné was a system for growing pure yeasts that we should probably learn more about.]
Finally, the figures above show that addition of insufficient amounts of acid results in a reduction in alcohol yield. This fact, quite often observed, is difficult to explain. It is probably due to the action of organic acids liberated by sulfuric acid. Owen and Bond conclude from the foregoing that it is important to specify experimentally by laboratory tests, for each yeast race and for each molasses type, the acid needs of the must.
[If yeasts are stressed, they will produce acetate at the expense of alcohol. They can easily happen in higher pH environments. High pH heavy rums need strategies to reduce this acetate, beyond continuously neutralizing it.]
The measure of alcohol produced in the presence of variable amounts of sulfuric acid is a rather delicate method, the difference between the yield of the optimum solution and that of the solutions containing inadequate amounts of acid, being generally low. Another more practical method is to measure the acidity at the beginning and at the end of fermentation. [Δ acidity! There will be a laissez faire number and any acid above that is at the expense of alcohol, be it from yeast stress or bacteria.]
It has indeed been observed for a long time that the higher the acidity during fermentation, the lower the alcohol yield. Fernbach (1) in particular has shown that if the acidity produced by pure yeasts can vary with the races, it is always stronger when the liquid is primitively less acidic. It has long been accepted in beet distilleries that the increase in acidity should not exceed 0.2 gr., in good fermentations (Boullanger). [He may be referring to pH crash in unbuffered ferments. I have also heard elsewhere that 0.2 g/L is a rule of thumb you start from.]
(1) C. R. CLVI. 77, 1913.
Hildebrandt, in the special case of cane molasses, has found that the maximum alcohol yield is obtained when must pH is not modified during fermentation. Here are some of the results obtained by this author:

In the above tests, optimum pH is close to 5, but it is likely to vary between 4 and 6, depending on yeast races. The true criterion of optimum acidity is that the final pH does not tend to be higher or lower than the initial pH. The easiest and safest way to regulate must acidification will therefore consist in achieving the constancy of pH during fermentation. [Either a wider buffer or periodic adjustment. pH was a very new concept back then which opened a lot of doors.]
This is so when one wants to get maximum alcohol yield. If it is proposed to produce beverage alcohol, whose bouquet is a function of the secondary products formed during the fermentation, it is not the same.
When the pH is relatively low (4.5-5.0), the fermentation is fast and the rum obtained light. If it is high (5.5-6.0), a larger quantity of fatty acids and esters are produced. The eau-de-vie is more full-bodied, more mellow and finer. But fermentation is longer and tricky to conduct. In particular, it is important for the must to be sterilized beforehand in order to eliminate bacteria whose development interferes with that of yeast in low acidity environments.
According to Arroyo, optimum pH for rum production would be between 5.5 and 5.8, and titratable acidity between 1.5 and 2 cc of N/10 soda per 100 cc off must. However, if the must has not been previously sterilized (at least partially), it may be necessary to go down to 5.0 and even 4.5 to avoid contamination by bacteria and wild yeasts. In production of very full-bodied rums, where fission yeasts are used, which adapt to high acidity, the pH will be even lower. The distiller will have to determine experimentally what is the optimum pH for the particular breeds of ferment he uses and special manufacturing conditions (saccharine richness of must (1), fermentation temperature, type of rum, etc…). ###
(1) Yeast development is closely dependent on the alcohol – temperature – acidity complex. If temperature and saccharine richness are high, it is necessary to have a relatively high pH, otherwise yeast action will be paralyzed. In the case of high temperature and acidity, it is important to lower the sugar content of the must.
Arroyo also studied fermentation at pH maintained constant by neutralization of acids during fermentation. By this process, he obtained rums that were more aromatic, more mellow and quicker to mature. He attributes these results to the fact that the lower fatty acids (formic acid, acetic acid), which have a pungent taste, are eliminated in the form of salts, whereas higher fatty acids are less easily neutralized by the added alkalis and tend to form valuable esters chemically during fermentation and distillation; at the same time, formation of rum oil is increased when pH is high.
Here are some results obtained by the author above, adding liquid ammonia to the must during fermentation (fermentation temperature 27-29 ° C.).

Arroyo advises to operate industrially as follows:
The previously sterilized must is brought to pH 5.8-6.0, then 1 gr. of sterile lime carbonate per liter of must, avoiding stirring the liquid so as not to cause a change in pH; the carbonate is deposited at the bottom of the tank and has almost no influence on the pH, if one does not carry out agitation. The pH of the yeast footing is also adjusted to about the same value as that of the mash.
According to the author, this method would give a rum of high quality, very difficult to imitate. It is unfortunately difficult to apply because it requires work in aseptic environment, to protect the yeast against the occurrence of bacteria.
Nutrients and catalysts.
Cane juice musts often have a deficiency in certain elements necessary for feeding the yeast, and in particular nitrogen. Molasses, on the other hand, may contain an excess of mineral matter, which hinders yeast development, and the harmful action of which may be diminished by addition of antagonistic salts. Finally, use of small quantities of substances acting as stimulants or catalysts is likely to accelerate fermentation and increase alcohol yield.
Although so far only the use of ammonia salts and, to a lesser extent, phosphates has been of importance in rhumming, numerous tests have shown, however, that the benefit of various other substances acting on yeast nutrition.
Nitrogenous substances.
Sulfate of ammonia. — Sulphate of ammonia was already commonly used in the French West Indies at the end of the last century, usually at a dose of 400-500 gr for 1,000 liters of must. At present, we add, in Martinique, 100 to 300 gr of this salt per 1,000 liters In a vesou distillery, the most common proportion is 300 gr per 1,000 liters, while in a molasses rhummerie, sulphate of Am. is sometimes removed, especially when you want to get a full-bodied rum. It is about the same in Guadeloupe, where the dose of 200 gr sulphate of Am. is often used per 1,000 liters of molasses must. In Reunion and Madagascar, musts are almost always composed of Am sulphate.
In English Guiana 1 kg of Am sulphate is added per 1000 liters of must. The dose is lowered in the United States, for the work with blackstrap molasses (50-55% of sugars), 100 gr per 1,000 liters must at 16-18° Brix density. In the Magné process, only 250 gr of Am sulphate are introduced for 1,000 liters in the yeast propogation apparatus, 350 gr p. 1.000 in the intermediate tank and nothing in the fermentation tanks. On the other hand, in the work of Cuba’s invert syrups, much larger quantities of up to 3 kg per 1,000 lbs. to achieve maximum alcohol yield.
In Jamaica, sulphate of Am. is never used.
The addition of nutrient salts, by causing a more vigorous yeast development, makes fermentations faster and reduces dangers of infection by foreign ferments. However, there is an optimum dose, according to Pringsheim, between 0.004 and 0.008% assimilable nitrogen, above and below which fermentation is slowed down and yield decreased.
Proportion of molasses nitrogen is generally sufficiently high for the must to contain an amount of this element at least equal to the above optimum. It seems, therefore, that the use of ammoniacal salts in the fermentation of cane molasses may be dispensed with. Nöel Deerr, for example, observed that in the case of the Hawaii molasses examined by him, which contained 0.24 1.06% nitrogen, corresponding to 0.05-0.2% nitrogen in the mash, Am sulfate, had no influence either on fermentation duration or alcohol yield. [there may be a problem with that second to last set of numbers.]
Arroyo, however, considers that if the total nitrogen content falls below 1% in molasses, it becomes necessary to add ammonium sulphate (0.2 to 0.5% of the molasses by weight) to obtain a good fermentation in the rhummerie. Above 1% of total nitrogen, addition of this nutrient is no longer motivated. According to the same author, Puerto Rican molasses would have, with a few exceptions, a generalized deficiency of nitrogen. Optimum nitrogen concentration for musts at 18-21 Brix, containing 11.5 to 13.5% of total sugars, would be 75 to 100 mgr. N per 100 cc of must. [We can start to optimize by titrating for YAN like wine makers do, but be aware Arroyo also used liquid ammonia to strategically raise pH to maintain optimal enzyme activity.]
Cane juices, especially if they have been defecated beforehand, as well as battery syrups, generally have a deficiency in nitrogenous matter. Owen, for example, has shown that to give maximum alcohol yield, invert syrup mashes at 24° Brix density, required a contribution of 3 gr. Am sulphate per liter.
Kozo Suzuki, in Formosa, has found that the optimum amount of Am. sulphate, for vesou musts containing 8% sugar, is 0.5 gr per liter, if this nutrient salt is used alone. But when using bipotassium phosphate at the same time, the best results, as regards both alcohol yield rapidity of fermentation, are given by a dose of 1 gr. of Am. sulphate and 1 gr. of phosphate per liter.
According to Iwata, the most appropriate proportions for cane juice from the first and second mills are 1 gr. of each of the above salts per liter. For the juice of the third mill, a dose of 0.5 gr of Am. sulphate and 0.1 gr K phosphate is sufficient. As for the juices, which are richer in nitrogen and mineral salts provided by insufficiently ripe canes, damaged by parasites, or by “white tips”, they do not need to be supplemented with nutrients. More than half the amount of nourishing salts is required for the overweight canes than for normal canes. [Fascinating!]
Nitrogen determination in the raw material will provide interesting indications on the needs of the must for Am sulphate. However, comparative laboratory tests will be necessary to specify the optimum dose, which may vary with yeast race.
Use of ammoniacal salts has some disadvantages, when one wants to obtain a full-bodied eau-de-vie. It decreases the amount of higher alcohols produced. Most of these come from the transformation of must amino acids by yeast, which tends, when it has a more easily assimilated nitrogen source, not to attack the amino-acids. [very key concept]
Various nitrogenous materials have been proposed from time to time in place of Am sulphate. Although their use is not popular, it is interesting to say a few words, because of the advantages that they can present in certain cases.
Ammonia and various ammoniacal salts. — Use of ammonia has been advocated by Owen, for fermentation of invert syrups. If high doses required by these last come only from Am sulfate, fermentation begins satisfactorily, but, as a result of the accumulation of SO4 ions, it soon becomes languid and stops before the sugars are completely exhausted. To counter this inconvenience, the author proposes replacing half of the sulphate of Am by a corresponding dose of ammonia [hydroxide], which is added to the must when the fermentation is about in its middle. [another key trouble shooting insight]
In this case, use of Am phosphate or, what ever is cheaper, albuminoid nitrogen, in the form of dried blood or distillery bottoms for example, can also be advantageous. [I’ve never heard of this blood usage before.]
Use of ammonia, in the form of a 10% solution of NH3 [ammonium hydroxide], was also recommended by Arroyo for the preparation of starters. Ammonia accelerates yeast cell multiplication and is more effective than Am sulphate in lowering the pH, as shown by the tests below. The doses used were 4 gr. pure Am-sulphate (all at once) and 1 cc. pure ammonia (several times) per liter of must. [I think there is a logic problem in there. Ammonium hydroxide lowers the pH less and sulphate which is valuable for high pH full bodied rums.]

By using ammonia in solution in the fermentation of the main must instead of Am sulphate, the above mentioned author has also observed a reduction in the duration of the fermentation and an increase in the alcohol yield, at the same time as a decrease in the level of higher alcohols and an increase in that of other products. In the tests below, the fermentation was carried out at a temperature of 27-29 ° C.

Various other ammoniacal salts: carbonate, phosphate, Ammonium Hydrochloride, have been experimented with. Under ordinary conditions, they do not offer any clear advantages over sulphate, and, as they are more expensive, they are not to be recommended. It should be noted, however, that Azzi (1) in Brazil obtained maximum fermentation yields with cane molasses by use of Am phosphate at a dose of 150-200 gr per hl.
(1) Bol de Agricult. São Paulo XXXVI, 330, 1935.
Urea. — Lindner and Wüst, then Bokorny, observed that urea was an excellent source of nitrogen for yeast, which develops remarkably in a urine solution with added sugar. According to Zeller (2), addition of urine to the musts would determine an increase in fermentation activity up to 200%. This would be due in part to ammoniacal salts present, but especially to a stimulating substance soluble in alcohol, precipitable by insulin and whose action would be comparable to that of vitamin B of Gigon or biocatalyst Z Euler. Optimum dose of urine is 1-2%, and urine of the night is more active than that of the day.
2) Biochem. Z. CLXXII, 142, 1926.
Peptonized and autolyzed yeast. — Degradation products of albuminoidal substances (albumins and peptones, amino acids) are easily assimilated by yeast. On several occasions, it has been recommended to add the bottom of the vat, previously treated so as to carry out the peptonization or yeast autolysis.
Barbet obtained a fermentation improvement with beet molasses, by use of peptonized bottoms by addition of sulfuric acid under pressure.
The muddy yeast residue is boiled in a Krüger, for example, and distilled until the alcohol is exhausted. A dose of sulfuric acid corresponding to that required to ensure acidification of the normal must is then added; it is closed and brought to a pressure of 2 kg. The yeast cells are gradually dissolved. The acid mixture is then cooled and added to the must. It takes about 500 grams peptonized yeast per 100 kg molasses worked. [This is quite interesting and may be useful.] ###
In the Bauer process, peptonization is carried out by bacteria. The yeast is left to itself for 3 or 4 weeks in small vats, which receive daily the amount of yeast corresponding to consumption. [This sounds very much like muck and now we are seeing it relates to peptonization.] ###
Autolysis can also be carried out by keeping the yeast previously diluted in water in a vacuum at a temperature of 50° for a few days. [I tried this and it did not work for me.]
If you start from a brewing or bakers yeast, you can make a dough with it by diluting with distilled water. An equal weight of a saturated solution of white sugar is added.
Owen was able to obtain, by adding autolysed bottoms to cane molasses musts, appreciable surpluses, as shown by the figures in the table below:

Owen found that yield increases were most pronounced in the case of high-density, high-sugar musts. [This is from Owen’s 1940 text]
Kayser has shown that in presence of autolyzed yeast, there is greater production of impurities, especially higher alcohols. Here are some results obtained by fermenting a 12% molasses must with 25% vinasse with 2 rum yeasts, in the absence and in the presence of 2% autolysed yeast:

In another test with autolyzed rum yeast (2%), the same author obtained:

Proteolytic products resulting from autolysis act not only on the quantity, but also on the nature of the esters obtained. Alcohol produced by the autolysed yeast ball of the previous test had a very good taste and was more perfumed than that of the control ball. Ester compostion was as follows:

Finally, fermentation of the balls that had received autolyzed yeast started more quickly and ended earlier. Kayser therefore recommends the addition of autolyzed yeast in the case of fermentations made with certain pure yeasts, which are not very productive of higher alcohols, and also to activate fermentations. [Fascinating ideas here. I think I am correctly translation ballons to ball.]
It should be pointed out that the sludge from the fermentation tanks and the lees of the distillation apparatus, mainly made up of yeast cells, appear to play an important role in preparation of grand arôme rum. These materials, which are collected in the “muck hole”, constitute a source of nitrogenous materials, whose transformation, by yeasts and bacteria, would contribute powerfully to bouquet production (Allan). Similarly, in the manufacture of grand arôme rum in Martinique, yeast deposits, instead of being evacuated with each new fermentation, are kept in the vats: it is only when their level exceeds a certain height that they eliminate some of it. [A very unique insight about Martinique!]
Phosphates.
Phosphates play an important role in alcoholic fermentation. Various authors have shown that they stimulate in a very marked way the fermentative activity of the yeast (Delbruck, Elion, Young). Elion (1) in particular has found that monopotassium phosphate and neutral phosphate of ammonia determine an increase in the release of CO2, varying, in a given time, from 28 to 63%, depending on yeast race.
(1) Zent Bakt. Parast. XIV. 1893/.
However, although molasses musts are generally low in phosphoric acid, addition of phosphates has rarely yielded industrially profitable results.
Peck and Deerr, for example, were unable to achieve fermentation acceleration or increased alcohol yield by adding lime phosphate to Hawaii molasses feed, which contained less than 0.03% P2O5.
On the other hand, Owen and Chen found that Cuba’s invert syrups, to achieve maximum alcohol yield, required a relatively high intake of phosphoric acid: 1 gram of potassium phosphate per liter of wort at 20° Brix.
In industrial fermentation of final molasses from Puerto Rico, 250 to 500 grams of lime phosphate per 1,000 liters.
According to Arroyo, optimal dose of phosphoric acid in molasses fermentation will vary between 0.2 and 0.25% of P2O5. Phosphoric acid deficiency would be much less common in Puerto Rico molasses than nitrogen. An excess of P2O5, which suffers from an insufficiency of N, seriously hampers, according to observations of the same author, the good progress of the fermentation which could even be completely stopped in extreme cases. In such cases, addition of nitrogen, in an amount sufficient to bring P2O5 / N ratio to about 1/5 would restore normal fermentation immediately. Optimum phosphoric acid concentration for musts at 18-20° Brix, containing 11.5 to 13.5% total sugars, would be 15 to 20 mgr P2O5 per 100 cc of must.
Experiments carried out by Kozo Suzuki and Iwata in Formosa on cane juice showed that the best results were obtained by addition of 1 gram K phosphate per liter, with normal juices. Vesou provided by immature canes or those strongly attacked by insects do not require phosphoric acid.
Salts of magnesia, manganese, etc.
Various authors have pointed out the favorable action exercised on alcohol yield and on fermentation rapidity by certain metallic salts. It is, moreover, most often difficult to specify whether these intervene by supplying the yeast with a nutritive substance which is lacking in the must, or if they act as stimulants of zymatic function. This stimulation can itself be of a biocatalytic nature, or result from neutralizing action exerted with respect to certain toxic principles produced by yeast.
Use of sulphate of magnesia is often advised in fermentation of invert syrups and molasses of high saccharine richness. Owen and Chen obtained a slight increase in alcohol yield and a significant increase in fermentaiton speed, adding to Cuban invert syrup musts (at 20° Brix) sulphate of Mg, at a dose of 1.5 grms per liter. It is possible that, in certain cases, raw sugar materials, and particularly defecated cane juice, have a deficiency in magnesia, which makes it useful to add this element to musts.
Kayser observed that magnesium salts also act on secondary products of fermentation, notably by increasing the level of higher alcohols and decreasing the level of esters. This author obtained, in a fermentation test with and without phosphate of magnesia, the following results (in grams per hl) of alcohol at 100°):

Guanzon and Lopez found that Mg sulfate, employed at the concentration of 0.005 to 0.01 mol. (0.60 to 1.20 grams per liter), increased the alcohol yield of cane molasses (1) in proportions ranging from 2.46 to 8.88%. With the sulphate of Ca, used under the same conditions (0.68 to 1.36 grams per liter), alcohol yield was increased from 8.27 to 11.12%. The useful effect of Ca sulphate was decreased by simultaneous use of Mg sulphate. These results are due to the antagonistic action exerted by Ca-ions vis-à-vis the K ions and by MS ions vis-à-vis Na ions. In tests which they carried out, the above authors indeed observed a reduction of yield, when sulphate of Ca was employed in the same way with sulphate of K, and sulphate of Mg with Na sulphate. This last salt, used alone, gave lower yields than the controls. As the cane molasses often contain very high proportions of K or Na salts, addition of Ca or Mg sulphate to the must would counterbalance the harmful influence exerted on yeast nutrition by a physiologically poorly balanced solution. Ca and Mg, which have a pronounced antagonism between them, must never be used simultaneously. ###
(1) The molasses, diluted at 18 ° Brix, contained per liter: Ca 0.5825 gr. ; Mg 0.1464 gr .; K 0.4316 and Na 3.9590 gr.
Lasnitzki and Szorenyi (1) found that potash salts (KCL) greatly increased the rate of fermentation (by about 150%). Even in the case of cane molasses, which are, however, very rich in K, addition of potassium salts could in certain cases cause increases in yield. Guanzon and Lopez were able to obtain an increase of 1.01 to 3.69% in the balloons treated with K sulphate at a concentration of 0.005 – 0.01 mol. They attribute this to the antagonistic action exerted by K on metal ions other than Na, which are normally found in molasses. [SOS I’m not sure about “ballons” here. Does it refer to their small scale testing glass?]
(1) Biochem. J. XXIX, 580, 1934.
According to Sanzo and Pirrone (2), if seawater is added to glucose solutions, a marked acceleration of the alcoholic fermentation is observed (20 to 24% with 13% of water). This action would be more pronounced when the water was freshly collected. At a high dose (above 20%), sea water causes a marked delay in fermentation. Brill and Thurlow (3) have observed in the Philipine Islands, where molasses is often diluted with sea-water for must preparation, that alcohol yield diminishes proportionally with sea-water quantity, when it exceeds a certain rate. The yield of alcohol (% theoretical yield) of 71.6 in the case of molasses diluted with distilled water added with 3 equivalent of NaCl (0.48 gr per liter), fell to 63.4, when using 1/4 of seawater, and at 55.5, if only the latter was used.
(2) Atti R. Acad. Linell 161, XIII, 140, 1931
(3) Philippine J. Sc. A XII, 267, 1917.
Mitra (4) in California, found that Na, K, Ca and Mg chlorides, if they reach a certain concentration are more or less toxic to yeasts. KCL presented itself as the least toxic and NaCl as the most toxic.
(4) Univ. of California. Public. in Agr. Sc. III, 63, 1917.
Kayser and Marchand (5) observed that addition of manganese sulphate to a concentrated sweet must (1 gr and 1.5 gr of sulphate per liter to 24% of sugar) had the effect of pushing the fermentation much further and giving an increase of alcohol sometimes reaching 3%. The amount of glycerine and volatile acids is also increased, while fixed acids decrease. Lactate, acetate and nitrate of Mn behave substantially like sulphate, but with succinate and phosphate one observes, a greater disappearance of sugar and lower contents of alcohol than in the control. Addition of yeasts to manganese salts would, according to Kayser and Marchand, lead to more complete fermentations. Manganese phosphate increased the level of impurities, particularly that of higher alcohols and esters according to Kayser.
(5) Ann. Inst. Agr. 121 VI, 355, 1907.
More recently, Hildebrandt and Boyce, experimenting with cane molasses, found that the use of small amounts of manganese sulphate, copper sulphate and sodium cyanide yielded an increase in alcohol varying from 1 to just over 2%. Stimulating action was more pronounced and more regular, when instead of adding the metal salt to the normal must, it was added to the yeast starter, at a dose of 1 per 1000 to 10,000 in the case of sulphate of Mn, for 1000 to 3,000 in that of Cu sulphate and 1 in 10,000 in that of Na cyanide. The operation must be renewed with each new yeast starter, the action of salts not being felt beyond the second generation. This way of makes it possible to reduce to a small number the quantity of necessary salts, makes them relatively inexpensive for use in industrial fermentations.
Gimel (1) has observed that bismuth sub-nitrate and tin protochloride exert, at very low doses, a remarkable action on yeast activity. The tin salt especially, added to musts in the proportion of 1/10,000, produces an increase in alcohol yield of about 4% compared to the control. Fermentation is also faster. However, Peck and Deerr have not been able to observe an increase in yield by the use of tin chloride with cane molasses musts. Mn sulphate, at a dose of 1 gr per liter, gave them an increase of 1%, but only in the absence of ammonium fluoride.
(1) C. R. OXLII, 1924, 1908.
Lastly, Owen (2) observed that by a short exposure of cane molasses must to ultraviolet rays, fermentation speed and also alcohol yield were increased. Yeast fermentative power being increased, it would be possible to reduce the amount of yeast used by 24%.
(2) Food Ind. V, 252, 1934.
Use of antiseptics.
In the distillery, antiseptics are substances that promote alcoholic fermentation and hinder secondary ferments. In the broad sense of the word, sulfuric acid and other acids, mineral or organic, are therefore antiseptics. More particularly, however, are meant products which, added to the must in small amounts, that paralyze foreign ferments while allowing the work of the accustomed yeast.
The most used antiseptics in the fermentation industries are sulfur dioxide and hydrofluoric acid. We have also recommended formalin, copper sulphate, etc…
Sulfur dioxide, widely used in wine making, can not be used in distillery. It gives rise, in fact, by diatasic hydrogenation to hydrogen sulphide. This gas, as well as free SO2 combine with alcohol during distillation, to form sulphides and ethyl sulphites, whose unpleasant taste and smell depreciate the eau-de-vie. It is the same with sodium sulphite, which gives, by reduction, hydrogen sulphide.
Formalin is used mainly for cleaning equipment (vats, pipes, etc.). It has also been thought to add it to musts. At a low dose (100 cc of formaldehyde for 3,000 liters of must or 200 liters of yeast), it does not harm the yeast and protects diastase. Addition of 0.5 cc per liter makes it possible to obtain very pure fermentations and a more regular alcohol yield (Lange). Yeasts can readily acclimatize with formalin: this acclimation is accompanied by intensive production of an oxidizing principle, which transforms aldehyde into formic acid and acts as an antibody (Effront). According to Cluss, however, formalin is less advantageous in terms of alcohol yield than hydrofluoric acid and, according to Krassnikoff, it would hinder yeast development.
Copper sulphate gave Pozzi-Escot and Denys (3) interesting results in cane molasses fermentation. In practice, 100 mg of this salt per liter suffices to obtain pure and active fermentations.
(3) Bull. Ass. Chim. XL, 113, 1933.
Denys (4) proposed use of salicylic acid, at a dose of between 1/40,000 and 1/50,000 of the must volume. This product would have a powerful antiseptic action, especially with regard to acetic bacteria. At the same time, it would hinder action of oxidases and increase yeast fermentative power. The author claims to have been able to obtain, through salicylic acid use, yield increases of 35 to 40% in cane molasses distilleries in Paraguay. Fermentation is also accelerated. [#neutrogena]
(4) Bull. Ass. Chim. XLIX, 279, 1932
Hypochlorite lime (bleach) was recommended by Alliot and Gimel for preparation of starters (50 gr of hypochlorite per hl of must). This product accelerates, at low dose, cell multiplication and has a pronounced bactericidal action. On the other hand, it oxidizes sulphurous acid and sulphites, to transform them into sulfuric acid and sulphates [notice the chemical subtlety]. Its use can therefore be very interesting in the case of molasses obtained by the sulfitation process, which contain sulphites of Na, K, Ca, which may be reduced by yeast and provide products with an unpleasant odor. Bleach has been tested by Auffret in Guadeloupe, in cane molasses fermentations. At a dose of 0.5 mg Cl per liter, it gave a good alcohol yield, higher than that of a must with 125 gr of sulfuric acid per hectoliter.
According to Krassnikoff (1), treatment of molasses musts with chlorine stimulates yeasts budding, if the dose is not too strong. Cl allows oxygen to act on cell respiration and it probably modifies the medium in a direction favorable to yeast development.
(1) Bull. de la Dist. de l’ U. R. S. S. N 2, 1937.
Hydrofluoric acid and fluorides are virtually the only antiseptics widely used in distilleries, HF, which was first used by Effront, is a powerful antiseptic against bacteria, even at low doses: 5 to 10 gr of industrial hydrofluoric acid (at 30%) per hl of must are enough to stop any multiplication of these organisms.
Yeasts can be acclimated easily to bear large doses of HF, making them live in the presence of increasing amounts of this acid (up to 36 gr of HF per hl). According to Effront, in this acclimatization, the yeast is enriched more and more in lime, which immobilizes hydrofluoric acid in protoplasm in the form of insoluble Ca fluoride. There is a decrease in the levels of glycerine and succinic acid formed and an increase in the amount of alcohol. Yeast fermentative increases at the same time as intensity of multiplication is reduced. According to McNelly and McLean, fluorine strongly decreases the proportion of glycogen in cells. Acclimated yeasts maintain their acclimatization after a series of cultures. They are also able to develop in environments that are very different from each other and that in themselves are particularly unfriendly to them, but they do not support modifications of these environments (Lévy (2)). It is thus possible to carry out virtually pure industrial fermentations, without sterilization, by introducing into the musts a quantity of antiseptic which stops development of all ferments, except that of the acclimated yeast.
(2) Microbes et distillerie, Paris, 1900.
Certain salts of hydrofluoric acid, especially fluorides of Na, Am, Al, have the same properties as the acid itself. They are however less energetic to obtain the same result than with 5 gram from HF it takes 35 to 40 grams of sodium fluoride, for example, per hectolitre. [yeasts love free basing hydroflouric acid…]
Quantity of fluoride used has a considerable influence on yield (Perard), the optimum varying with must quality and yeast race. It will be as weak if the must is more acidic and poorer in nutrients. It is important, therefore, to determine experimentally the optimal dose for a given medium and yeast race employed. Even with un-acclimated yeast, addition of fluoride improves yield, provided however that the dose is low and does not exceed 1 to 2 grams per hl. In industrial practice, fluoride is usually used at a dose of 10 grams per hl, but one would sometimes gain for this to be increased. In an experiment conducted by Pérard (3), an alcohol yield of sugar of 58.90 with 10 grams per hl from fluoride was raised to 61.30 with 20 grams and to 62.60 with 40 grams of antiseptic.
(3) C. R. 5. Cong Int, des Ind. Agr. 802, 1937.
Use of hydrofluoric acid and fluorides is particularly indicated in distilleries producing industrial alcohol. In the rhummerie, it has the disadvantage of greatly reducing the rate of secondary aromatic elements and gives products that, from an organoleptic point of view, tend to approach neutral alcohol.
In some rum producing countries, however, these antiseptics are used on a certain scale. This is particularly the case in Guadeloupe, where more often one adds to molasses and vesou musts, a dose of fluoride Na variant, depending on establishments, between 5 to 20 grams per hectolitre. Fermentations are pure and fast (24 to 48 hours), but the rum impurity coefficient goes down in some cases below 200 and rarely exceeds 350 (Auffret). Level of volatile acids is particularly low. [how sad!]
In English Guiana, sulfuric acid is also replaced by Am fluoride in some distilleries (N. Deerr).
There does not seem to be any reason to recommend use of true antiseptics in rhummeries, since the increase in yield they can achieve does not compensate for the decrease in the quality of the product.
Addition of various products to musts
In the primitive processes of fermentation of cane juice or molasses, in order to produce rum or wine for direct consumption, leaves and tree bark, fruit juice, etc., are often added to musts. On the other hand, in recent times, various experimenters have studied the action of inert substances on fermentation (coal, bagasse, etc.) and obtained in some cases results worthy of attention.
Leaves and bark.
Charpentier de Cossigny wrote at the beginning of the nineteenth century about production of “sugar spirits”:
“I said in my Memoirs on the Manufacture of Sugar Spirits that Indians make two kinds of it with jaggery, which is a coarse saccharine substance that they extract from coconut palms or palm trees; they mix it with water, ferment it and distil it. One, made without care, is very bad; it has a very disagreeable smell, and is consumed only by lower classes; the second is called “araque-pattai”; it is more expensive and preferable to the other. It takes the name of a species of acacia that provides a very beautiful gum, yellow and transparent … The Indians remove the bark of this tree they call “pattai” (1) and add it to the liquor that they distill. I tried it on fermented vesou; this bark gave it quality…”
(1) The plant known nowadays in Malaysia under the name of “pattai” is the Parkia speciosa Hassk.
“The leaves of the tree named attier in the Indies and African Islands, and apple cinnamon tree (2) in Santo Domingo, mixed with grappe in the still, also give good taste to the liquor that is distilled.”
(2) Annona squamosa L. [By appearance, this seems like a cousin of the Jackfruit.]
“Madecasses de Foule-Pointe, located on the east coast of Madagascar, make an intoxicating liquor with cane juice, before the fermentation, they add the leaves of ambrevade (3), they have a little bit of bitterness, but they have the property of increasing the strength of liquor …”
(3) Cajenus indicus Spreng. [Keep in mind, they use the leaves.]
We have reproduced this quotation, since the indications contained therein have been repeated many times in the works or articles relating to the manufacture of rum, until the last few years.
According to Gibbs, Holmes and Agcaoili, natives of the Philippines employ in manufacture of basi, a fermented drink made from cane juice, leaves and fruits of samak (Macaranga tanaria Muell-Arg.), and in tuba, another fermented drink also based on vesou, crushed roots of lankawas (Cordyline terminalis Willd). [possibly Alpinia Galangal]
Guanzon and Megia (1) found that dried leaves of samak added to the must have an effect not only on the bouquet, but also on alcohol yield, which is significantly improved. They obtained the yields below (% theoretical yield), adding to a must of cane molasses at 20° Brix, 1/2 gram per liter leaves of various plants (air dried): Macaranga tanaria, Macaranga bicolor, Cajanus indicus, Areca catechu. The sample with Am sulfate received 1 gram of this salt per liter.

(1) Sugar News XIX, 227, 1938.
These figures show that the plants studied give results comparable to Am. sulphate as regards alcohol yield, but that fermentation is slower. When Am sulphate is very expensive or even lacking due to special circumstances (war, etc ..), we can consider replacing it with local plant leaves, preferably those of legume leaves, rich in nitrogen. The authors above could not specify to which constitutive material the stimulating action of the leaves used was due.
Jacquemin (2) studied development of aromatic principles by alcoholic fermentation in presence of certain leaves. Assuming that the characteristic fruit flavor was due to glucosides developed by the leaves (even when they do not have their own perfume, as in the case of apple trees for example) and passing thereafter in the fruit, where they undergo a doubling of glucose and an aromatic principle, he added apple and pear leaves to a sweet liquid which he fermented by means of a yeast not giving by itself a bouquet. He was able to note that during fermentation, the liquid gave off a pronounced smell of fruit and that the eau-de-vie obtained had a fine bouquet of apple or pear. Yeast, like the plant cell, has the power to split, by means of diastases, glucosides that are placed at its disposal. Certain aromatic products were very volatile and largely evolved during fermentation: distillation before the complete end of the alcoholic fermentation allowed a clearer and more pronounced bouquet.
(2) C. R. CXXV, 114, 1897 : CXXVIII, 369, 1899.
Jacquemin even observed, by immersing vine leaves, from various grape varieties, in musts of identical composition, that liquids obtained had different bouquets fermenting under influence of the same yeast. Introduction of whole or minced leaves into grape musts, however, gave them an unpleasant taste reminiscent of the dried leaf, which partly masked the fragrant principles engendered. The author was able to overcome this disadvantage by preparing, by diffusion and concentration, syrupy extracts of leaves. He concludes from experiments made on an industrial scale that the addition of extracts of grape varieties of grand crus, even at the minimal dose of 1/1000, would significantly improve the quality of wines and would be a valuable adjunct for pure yeast vinification.
In rum production studies, addition of fruit juice to must is quite often reported: sour oranges (Citrus aurantium L.), lemon peel (Citrus aurantifolia Swingle), pineapple (Ananas comosus (L.) Merrill) etc. These juices are mostly used by small manufacturers to start their first tanks. They act mainly on yeast and acidity that they bring to the must. Sometimes they also enrich the eau-de-vie with aromatic products, especially when added to the already fermented must. For example, in Jamaica, to give the rum a fruity aroma, pineapples and guavas are sometimes introduced into the still boiler (Thorpe) (1). [I’ve heard anecdotes of citrus rinds being added to cachaça starters to provide a physical footing for the yeast to grow and possibly beneficial actions by citric acid.]
(1) A Dictionary of applied chemistry V. 716. London 1921-26.
Addition of sugarcane pieces to musts is also mentioned by ancient authors in Jamaican rum manufacture. [These may be the infamous rums canes which have aroma beneficial infections.]
Let us note finally that in preparation of the ragi, yeast starter used for manufacture of Batavia Arak, enter various aromatic plants: garlic, galanga, cinnamon, ginger, anise, etc ….
Carbon and inert materials.
Action of inert materials on fermentation has been reported for a number of years. As early as 1913 Söhngen (2) studied the influence of filter paper, clay and garden soil and found that it stimulated fermentation. Neuberg (3), then Abderhalden (4), noted favorable action of colloids, the latter having shown that in the presence of bone black, larger quantities of glycerine and, above all, of aldehyde and acetic acid were formed, which is absorbed in part by the carbon. Ivekovic (5) attributed the accelerating action of bone black in part to absorption of toxic substances that retard fermentation and partly to a greater release of CO2. [Jamaica was using bone black as early as the 1840’s according to W.F. Whitehouse.]
(2) Zent. Bakt. Parasit. XXXVIII, 621, 1913.
(3) Biochem. Z. LXXXVIII, 145, 1918 ; OXXI, 215, 1921.
(4) Ferment Forschung V, 89, 1921; V. 255, 1922.
(5) Biochem. Z. CLXXXIII, 451, 1927.
According to Owen and Denson (6), stimulating action of vegetable carbons on cane molasses fermentation, though already pronounced with musts of ordinary sensibility, is particularly marked in high density solutions. It is also relatively more accentuated, when H ion concentration is lower and farther from the optimum suitable for yeast [high pH]. Carbon would intervene both by facilitating carbon dioxide evolution, by accelerating conversion of acetic aldehyde to alcohol and by stimulating yeast cell multiplication. This stimulation is attributable, on the one hand, to absorption of toxic substances in the medium and on the other hand probably to a catalytic action exerted by carbon.
(6) Zent. Bakt. Parasit. LXXVII, 481, 1929.
Pulverized carbon has been used industrially in a molasses distillery. Lamont (7), for example, reports his employment at the Cucau plant in Pernambuco, Brazil, and the very satisfactory results obtained. Wort, prepared from cane molasses and having a density of about 14° Brix, is supplemented with spent refinery carbon (Suma-Carb, Carboraffin, etc.) at the rate of 6 kg per 22,000 liters of liquid. Fermentations are fast and finish abruptly: tanks are ripe after 26 hours, instead of 36-40 hours, when one does not use carbon. As for alcohol yield, from 4.41% previously, it was raised to 5.35%, which corresponds to a 22% increase. It was further found that the amount of carbon needed varied with the season, cane variety and weather conditions.
(7) Int. Sugar 3. XXXII, 229, 1931.
Cane bagasse.
Recovery, by their transformation into alcohol, of sweet substances contained in bagasse, has retained interest for a long time. Already in 1862, Hugoulin (1), Navy pharmacist, recommended placing bagasse coming out of the mills into large closed cylinders, provided at the bottom with a steam supply pipe and at the top of an exhaust duct. Once the bagasse fermentation is complete, steam is conveyed which entrains alcohol formed.
Landes (2) suggested, in 1899, to ferment bagasse in open wooden vats. “Application of this process to bagasse fermentation would be”, he wrote, “a happy innovation. It would recover sugar contained in bagasse without having to intervene, which is not possible for agricultural rhummeries. The difficulty is to have an alcoholic fermentation fast enough that cellulose fermentation has barely time to start. It will still be possible to iron the bagasse at the mill, to express most of the fermented juice it contains, which will make it fit to be used again as a fuel”.
But it is only relatively recently that the question of bagasse fermentation has given rise to specific experiments by Owen and his collaborators.
First tests, carried out by Owen and Bennett (3) in 1925, showed that fermentable sugars contained in bagasse (5% on average) could be converted into alcohol. On the other hand, addition of bagasse to molasses musts tended to accelerate fermentation, but did not always result in an increase in yield.
Tests undertaken in 1928 by Owen and Denson led to the following conclusions:
Addition of bagasse to molasses musts increases alcohol yield, often in very appreciable proportions: by using 100 grams of bagasse (containing 5.12% of reducing sugars) per liter of molasses must at 15° Brix, yield increases were obtained ranging from 0.6 to 9% (5-6% on average). It also speeds up fermentation which is reduced by at least a third in the case of ordinary musts at 15° Brix, and in much greater proportions if the must is very concentrated. Thus, a molasses solution at 36° Brix, supplemented with 25 grams bagasse per liter, gave in 48 hours, more alcohol than the control after 120 hours. To produce its full effect, bagasse must be added at the beginning of fermentation. There is no need to sterilize or divide it beforehand. On the other hand, if seeded with yeast before mixing with the molasses solution, fermentation is faster and alcohol yield is higher. [My understanding is that bagasse may increase methanol in ways they were not able to detect back then.]
Bagasse intervenes mainly mechanically, by facilitating carbon dioxide release, as well as yeast distribution inside the must. But its stimulating action is also due to other unknown causes, because bagasse still acts when, placed in a porous container immersed inside the must, it is not in direct contact with the liquid.
Bagasse does not seem to have been used so far in rum production. Tanks used in distilleries producing grand arôme rum in Jamaica are half-filled with bagasse, but only in order to facilitate acid fermentation of products which are poured into them.
[I think that first line means it was only used for either industrial alcohol or only in laboratory research. Bagasse in the trash cisterns of Jamaica may very well be like cork particles in vinegar generators at wineries. It becomes a physical structure for bacteria to grow on.]
1 thought on “Kervegant Chapter V Preparation of Rhummerie Musts”