[I just received a very high end automatic titrators and will be revisiting all this soon.]
[This requires some major updating, especially about translating historic data tables, as I’ve learned a lot since. But I need to wait to move into the house I’m buying.]
A current project is adding acid and ester titrations to my birectifier and model fermentation work. In theory these are the two most simple titrations you can perform, but there is still a lot to learn if you have no chemistry background.
Titratable acidity (TA) will be valuable for looking at distillates because we are finding a strong correlation to heavy distillate quality and noble volatile acids. What we find in the distillate of role models can give us big hints at what was in their ferment and what we will need in ours. This titration skill set then segues right into ferments. pH may not tell enough because of the buffered nature of distillery ferments and we may need TA to untangle what we are actually creating in the ferment. The Δ of TA may also help us examine the contribution by bacteria both positively and/or negatively.
Additionally, in my currently limited understanding, measuring high alcohol concentrations with a pH meter can dehydrate the probe and effect results, possibly also causing instrument damage. Measuring TA allows us to add water, reducing the proof, while not effecting the measurement. TA may help us monitor the trajectory of spirits maturity.
Ester determination is an extension of measuring TA. First you titrate for TA and thus produce an essentially neutral sample**. Then you add a known but excess amount of sodium hydroxide and heat the sample with full reflux (for an hour or two) to the split the esters. As they split, the acids will join with the sodium hydroxide. Backwards titration with an acid will measure the excess amount of sodium hydroxide and thus also how much was absorbed by the acids from the esters. This is also typically performed in conjunction with a blank to help prove the backwards titration and increase confidence in results.
If we mix together the first three fractions of the birectifier, where roughly all the ethyl acetate is located, we can get a number we should be able to subtract from the total ester number to see our ratio of ordinary (ethyl acetate) to extraordinary esters (high value, long chain). We can use this to evaluate the quality of our process. Ethyl acetate is known to contribute the vast bulk of all esters in a spirit and hopefully we can generate numbers reliable enough to create a ratio. Rafael Arroyo explored this ratio and gives us some guidelines. Ethyl acetate is also a integral indicator of maturation because of how it increases until it plateaus. Monitoring it may provide insights about spirit maturation.
Before we even begin we should be able to learn the math behind the calculations and look at Arroyo’s numbers.

This table shows examples of the titrations Arroyo used. Keep in mind these figures do not represent all of the volatile acidity because much is left in the birectifier stillage. However we can still examine these numbers and understand what volumes and likely what concentrations of sodium hydroxide were used to get the result. Revealing this will let us know what to buy to duplicate the work.
Presently, nearly all professional life sciences’ titration work is done with automatic titrators that have built in calculators. They have micro fluidic pumps that keep dispensing sodium hydroxide until an end point measured by pH. Sadly, these are beyond the reach of most distillers. Fast and accurate but expensive. They could prove quite valuable if we learn to use the data.
In the distant past, things were a little like MacGuyver. pH was not used so titration was conducted until an end point was reached with an indicator like phenolphthalein which works by changing color. The standardized solutions would also have to be made yourself which takes certain knowledge. Titrants dispensed where measured with extremely tall graduated glass burettes. Cheap and clever and time consuming.
In the pragmatic present what we will do is use a modern pH probe (with a big emphasis on calibration) and a magnetic stirrer. To dispense the titrant into the analyte, we will use our automatic pipettes (screwing down the plunger to slowly dispense). First we will weigh and zero a small amount of titrant, and as we take from that with our pipette, we will measure what we subtract (and return unused). Knowing the density and the weight will tell us the volume of solution used and help our calculation. We will buy modern standardized titrants at concentrations optimized for our task.
Titrants come in concentrations such as 0.1 N or 0.1 M where N stands for normal and M for molar. In our simple titration these become the same:
You can convert from molarity (M) to normality (N) using the following equation: N = M*n
where n is the number of equivalents.
Note that for some chemical species, N and M are the same (n is 1). The conversion only matters when ionization changes the number of equivalents. (Reference: Quora)
This will be very important to know so we can confidently buy titrants because some are labed 0.1 N and some 0.1 M. We don’t need to be full on titration generalists, but rather just experts in our two basic titrations.
From Arroyo’s data———/4 ml of 0.1 N NAOH .05 N .02 N
Fraction 1: 4.27 mgs. 1.0675 mgs 0.1779 ml 0.3558 ml 0.8895 ml
Fraction 2: 2.67 mgs. 0.6675 mgs 0.11125 ml 0.2225 ml 0.55625 ml
Fraction 3: 2.67 mgs.
Fraction 4: 9.34 mgs.
Fraction 5: 16.01 mgs. 4.0025 mgs 0.6671 ml 1.3342 ml 3.3355 ml
Fraction 6: 6.23 mgs.
Fraction 7: 5.78 mgs.
Fraction 8: 3.56 mgs.
These figures are all per 100 ml of distillate even though only a 25 ml sample was measured. This means each needs to be divided by 4. This great manual (page 11) lets us know that 1 ml. of 0.05 N NaOH is equivalent to 0.003g of acetic acid.
Above, I did not calculate all the figures, but presented the high and low to see how much volume we accurately need to measure from commonly available concentrations of NaOH. Distillates are very likely at the low range of TA so we need to buy as low an N value as is available so our volumes are as easy to measure as possible. I am fortunate to measure using an analytic balance, but others may get away with a far less precise scale.
To arrive at my numbers, first I divided Arroyo’s mg figure by 4 to see how much acid he was looking at in his 25 ml sample. Then I divided by 3 because every ml. of 0.05 N NaOH is equivalent to 3.0 mg of acetic acid. Then I either divided by 2 or multiplied by 2.5 to reveal the volume of the other common concentrations (half as concentrated or 2.5 times as concentrated).
When we move on to esters in Arroyo’s chart, we see a much different set of numbers. They jump dramatically, possibly meaning we’re going to have an easier time with the volumes or we could consider titrants more concentrated than 0.02 N.
From Arroyo’s data————— /4 ml of 0.1 N NAOH
Fraction 1: 238.48 mgs. 59.62 mgs. 6.775 ml
Fraction 2: 42.24 mgs.
Fraction 3: 7.04 mgs.
Fraction 4: 35.20 mgs.
Fraction 5: 42.24 mgs.
Fraction 6: 28.16 mgs.
Fraction 7: 28.16 mgs.
Fraction 8: 28.16 mgs.
Keep in mind, we are also back titrating. First we are going to add a precise but excessive amount of NaOH then we are going to back titrate with either sulfuric or hydrochloric acid to reveal the difference.
From our handy guide (page 12), 1 ml. of 0.1N NaOH is equivalent to 0.0088g of Ethyl acetate. The guide protocol has already jumped to a higher concentration for the task of splitting the esters. We see that we would need at least 7.0 ml (working in round numbers) to exceed what the reaction requires for fraction 1. Reaction kinetics may even encourage significantly more. Working from a 50 ml sample of wine, the guide uses 10 ml of 0.1 N NaOH.
We will likely be working from either 25 ml of absolute alcohol samples of the complete rum (roughly 50 ml) or 75 ml samples (fractions 1,2,3) taken from the birectifier which are derived from 100 ml of absolute alcohol. When we create a ratio of both results we will have to be in mgs per 100 ml.
Something else to note is our guide back titrates from 0.1 N sulphuric acid.
From the guide protocol’s words about the blank:
To the neutralized distillate from the volatile acidity determination (Sec. 5.2.1), add 10ml of Std. NaOH and reflux on a steam bath for 1 hour. Cool and back titrate the unspent alkali against standard sulphuric acid. Carry out a blank simultaneously taking 50ml of distilled water instead of distillate in the same way. The difference in titer value in milliliters of standard sulphuric acid gives the equivalent ester.
Another method from the A.O.A.C. refluxes for 2 hours. Back titrating a blank means we only have to keep track of the difference in NaOH. This also means that we really only need two numbers to get us through the process:
1 ml. of 0.05 N NaOH is equivalent to 0.003g of acetic acid
1 ml. of 0.1N NaOH is equivalent to 0.0088g of Ethyl acetate
This heating bath requires the 500 ml glas-col mantle borrowed from the birectifier, a double neck 500 ml boiling flask and a 500 mm air condensor plus two thermometers (and 24/40 adapters). The heating can be controlled by the PID function of the birectifier’s custom Auber heating controller. The PID thermometer goes into the second neck of the flask and is set just below the boiling point of the sample, accounting for its alcohol content. Any vapor generated will be condensed by the air condensor and a second thermometer at the top will prove that it is at room temperature. The sample can be made to gently boil with complete condensing while using no liquid coolant. Pumice boiling stones can ensure there is no super heating.
Hanna Instruments just released a new generation of pH probes that I am excited to work with. They interface with your iPhone or iPad providing an easy to use interface you can screen shot and share. They are constructed of a glass body so you can examine the inner workings and view whether the probe is properly hydrated. The app interface gives a very intuitive way to read the results and calibrate the probe. With pH probes, calibration is everything and these should be done at three points (make sure you buy all three!).
I purchased the Hanna edge tablet for their Halo blu tooth probe, but had annoyances synchronizing it to the probe while my iPad worked effortlessly and the iPad interface was much superior. Hanna sells some electrode holders that make raising and lowering the probe for titration quite simple.
I would love to know more of the what ifs and techniques of calibration. My probe was slightly off of calibration, but I am not informed enough to know if the magnitude was normal or abnormal.
As the rum renaissance rolls on, to my knowledge, no small distilleries or super enthusiasts are set up to perform ester determinations. As we wade into this, one thing that must be recognized is that we are working with an antiquated technique and probably cannot use the data in any kind of official review or proclamation. All that we can say is hmm… and ask further questions. Nothing is finer than rum as we make it! But if you are learning rum making, you should always pick role models!
An interesting thing we can do is start to correlate our present day sensory experiences to these ester determinations and then back to historic data. We can reference Peter Valaer’s Foreign And Domestic Rums (1937) and see how beautiful new products compare to what was being bottled in the 1930’s. This is important as we strive to make sense of the grand arôme rums and how they were blended before bottling now that they are making a comeback. How does the most exciting rum of the market compare to historic data? With esters, bigger is not necessary better (there is rum oil to consider!). Can numbers guide us?
Will we gain insight from the ratio of ordinary ethyl acetate to extraordinary long chain esters? The point of a grand arôme rum may be to beat the typical ratios even though they drag along a large surplus of ethyl acetate. They possibly also have a take a certain shape to maximize rum oil.
Page 6 section 4.4 is worth referencing to understand how the electrode works. Its a little bit surprising. On page 7 we get some hints on what to expect when calibrating:
Standardization is normally done for acidified foods with pH 7.0 and pH 4.0 buffers. This gives an accurate reading within the pH region of interest, pH 4.6. Then the pH of 9.18 standard buffer should be measured. If the electrodes and pH meter are working properly, the pH reading on the pH 9.18 buffer should be between pH 8.88 and pH 9.48.
Section 4.6 gives some important advice. The electrode can be rinsed and blotted but never wiped! Even a tissue can damage the surface of the hydrogen ion sensitive glass membrane.
One page 9, section 4.8.1, we see a little bit more of why you can end a titration at pH 8.2 as an alternative to pH 7.0. The last increments of NaOH also make the pH rise more significantly. 8.2 is also the pH at which phenolphthalein changes color. Acid titration of clear liquid where the color change is observable can even be conducted without a pH meter, but I don’t yet understand the degree of accuracy. The last sentence of that section states that even with a pH meter, the titration should be carried out to a pH of 8.2. ***
*** The Khan Academy gives the best explanation of why the equivalence point is often higher than pH 7.0. The article is well worth reading but essentially the salt itself retains a charge which depends on if it is composed of a strong acid, strong base, a strong acid, weak base or vice versa. Our titrations are typically of weak acids (the analyte) while NaOH (the titrant) is a strong base so our salts will retain an alkaline charge pushing the pH at the equivalence point above 7.0. It thus makes sense to end our titrations at 8.2.
Section 4.8.2 on standard Sodium Hydroxide (NaOH) solutions is quite illuminating.
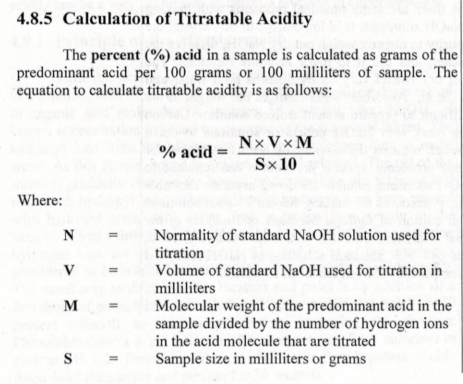
Section 4.8.5 gives more details on the formula for titration than generated the numbers we used above. Quite interesting.

A great fruit juice protocol is here. It reinforces the idea of titrating to an 8.2 pH end point and gives more data on the milliequivalent factor to convert to other acids besides acetic.