The first 50 pages start here as well as some ideas about helping along with the translation. In geographer Guy Lassere’s 1949 review of this remarkable text, we raise the question: if we take this book from obscurity, make it accessible by translation and digitizing, how much value will be unlocked for the rum industry? A single important lost text. A public work that you can build private enterprise on; how valuable is it? How many ships will it launch? We are continuously finding that there is much to be reconciled in the rum world.
This only made it from 51-80 (instead of p. 100) because WordPress really ground to a halt as the document length grew and the images added up.
[The very first half paragraph here was grouped with pages 1-50]
Cases of this nature have been reported particularly in Egypt, affecting molasses stored in silos dug in the ground (Habif, Neuville). A sample of charred molasses, examined by Habif (1), had the following composition:
(1) Bull. Ass. Chim. LIV, 809. 1937.

Prinsen-Geerligs also cites examples of bursting of vacuum boxes, during the preparation of molasses solidified in Java, as a result of spontaneous decomposition during the concentration.
A more attenuated form of froth fermentation is characterized by a production of co2 which not only produces scums on the surface, but also forms more or less deep pockets inside the molasses tanks. Sometimes granular deposits of a carbonaceous material similar to that indicated above are formed. In the case of syrups and table molasses kept in closed boxes, the pressure exerted by the carbon dioxide may be sufficient to cause the bursting of the containers (Browne).
It has long been recognized that froth fermentation of cooked masses and molasses was due, not to the activity of microorganisms, but to purely chemical reactions.
Prinsen-Geerligs hypothesized that it was caused by the spontaneous decomposition of unstable products resulting from the action of lime on sugars, and more particularly on reducing sugars, during the clarification of juices. Browne attributed a special role to glucic acid, whose lime salt readily absorbs oxygen from the air by producing formic acid and other products, with a great deal of heat. In its crystalline form, this acid can partially sublimate by the action of heat, finally giving compounds of dark color, rich in carbon. In the presence of organic impurities, it decomposes spontaneously.
It has been suggested that the reaction between amino acids and reducing sugars, which gives rise to very unstable compounds (Maillard), may also be involved in the spontaneous decomposition of molasses. This phenomenon, however, would be secondary and limited to the onset of decomposition, according to Browne, the transformation continuing to occur after the disappearance of amino acids.
The slow deterioration of molasses during their storage is generally regarded as a slower form of frothy fermentation (Prinsen-Geerligs, Browne).
The main causes that favor the latter appear to be the high concentration of molasses and the high temperatures during manufacturing and storage. Neuville (2) reports, for example, that molasses with 93.35 Brix, obtained in a sugar factory in Egypt in 1938, underwent a particularly rapid decomposition, while others having the same origin, but Brix only reached 90°, preserved without any alteration. In Java, it is considered that the temperature during the manufacture of solidified molasses should not exceed 75°, otherwise there is a risk of spontaneous decomposition. According to Honig (3), if during their storage the molasses stored at 30-35°C undergo a 2-3% reduction in fermentable sugars per year, a rise in temperature of 10° would be sufficient to quadruple the losses in certain cases. It is advisable, therefore, to cool the molasses before sending them to the preservation tanks and to paint them in white to reduce heat absorption.
(2) Proc. 6. Cong. Int. Soc. Sugar Cane Techn. 971, 1939.
(3) Proc. 5 Cong. Int. Soo, Sugar Sane Techn. 228, 1935.
In some cases, however, the reduction in the saccharine richness of molasses, is sometimes accompanied by various modifications of flavor and odor (molasses, tart or sour, moldy, etc.), is due to the intervention of ferments. Ashby, in Jamaica, has found a wild yeast (Torula) that can ferment molasses at 90° Brix. Tempany found the intervention of bacteria and molds in the molasses ripening of the Lesser Antilles. Owen, Church, Hucker and Pedersen have encountered many yeasts, bacteria and molds in molasses, some of which attack sugars in relatively high concentrations.
Some molds (especially Aspergillus repens) are able to determine the inversion of sucrose in solutions of 65 and even 70° Brix (Owen, Van Der Bijl). On the other hand, the bacteria would exert an appreciable action only at concentrations lower than 60° Brix. It would be the same, according to Owen (1), of most Torula encountered in molasses and whose activity would be limited mainly to the destruction of invert sugars. This last author gives the following example of the modifications undergone by a molasses first of Cuba, diluted to 71° Brix and sown with a Torula:
(1) Sugar XXXVIII, No 9, 22, 1943.

Under normal conditions of preservation, the losses resulting from the action of the various ferments are generally of little importance. Absorption on the surface of the molasses may be sufficient to allow the growth of mold and the consequent production of some oxalic and citric acids, but the molasses layer attacked, usually remains very thin and represents only a small part of the total volume.
It is quite different when the tanks remain open in the open air or consist of pits dug in the ground. In this case, as a result of the supply of rainwater, the dilution of the molasses may be such that the butyric bacteria are able to develop and give birth, if the storage is prolonged, to significant amounts of butyric acid.
To reduce the losses of sugar caused by microorganisms and to prevent the production of unpleasant aromatic substances that can then pass into the rum, it is important to concentrate molasses sufficiently (85-90° Brix) and to store them in closed tanks. Tubs, pipes and molasses tanks should be properly disinfected.
Value as fermentation material
The value of molasses as raw material for the production of rum is very variable and, in general, significantly lower than that of cane juice.
During manufacture and during the subsequent storage of molasses, sugars undergo chemical or biochemical transformations, which make them less easily fermentable (carameliform substances, glutose) or lead to the production of substances (formic, butyric, oxalic acids, etc.) constituting, at slightly high doses, poisons for the yeast.
On the other hand, the ash content, often very high, can hinder the functioning of yeasts, as found by various authors (Reindel and Frey (2), Arroyo, etc.). The equilibrium of mineral matter, dominated by salts of potash and lime, which in some cases (cane from salty soil) is very rich in Na chloride, sometimes leaves much to be desired. There are certainly cases where molasses musts are not a physiologically balanced solution. (1)
(2) Z. Spiritusind. LVII, 237, 1934.
(1) A solution in which the toxic properties possessed by the various salts, if they were alone, are neutralized by the presence of other antagonistic salts. The type of physiologically balanced solutions is seawater.
The most suitable molasses for the production of rum are those which have a specific weight and high levels of sugar, nitrogen and phosphoric acid, together with a small amount of ash and gums. The aroma is also of great importance, the presence of unpleasant smells and flavors affect the distillate. Fresh molasses always produce better rum than those that have been stored for a long time. Older molasses have a higher viscosity and often give a blackish scum, which is usually accompanied by a large decrease in alcohol yield.
Arroyo gives the following examples of molasses with different qualities from the point of view of rhum fermentation.

These molasses gave, in 3 fermentation tests at different concentrations, the following results:

According to Arroyo, a molasses can be considered excellent, when the quotient total sugars: ash is equal to or greater than 6.5/1: as good, when it is between 6.5/1 and 4.5/1, and as very mediocre, when it is less than 4.5/1. Nitrogen content is sufficient if the total N content is 4% of the molasses by weight; below, it is important to add Ammonium sulphate. As for phosphoric acid, its optimum dose is between 0.2 and 0.25% in P2O5 [Phosphorus pentoxide]). The P205: N ratio should be about 1/5; an excess of phosphoric acid relative to the nitrogen greatly hampers the smooth operation of the fermentation.
The richness of molasses in microorganisms is generally between 10 and 25% of that of cane juice. These microorganisms are represented by bacteria (mainly subtilis, mesentericus and butyricus groups), molds and yeasts (usually of the Torula type).
According to Church, the amount of bacteria in the molasses at the centrifuge outlet can vary between 10 and 2,000 per gram, while fungal-mold spores reach at least 40,000 and in some cases up to 200,000. Owen found that in the final molasses, the number of microorganisms is likely to reach a maximum of 100,000 per gram.
Some cane juice bacteria can withstand the spores at the high temperatures of sugar manufacture and are then found in molasses, but most of the microorganisms encountered come from infections during sugar turbination, manipulation and especially the conservation of molasses.
These microorganisms generally play only a minor role in the alcoholic fermentation of musts, the molasses dilution rate being large enough to reduce their concentration to a small number on the one hand, and the acidification of musts hindering their development on the other hand.
To achieve good conditions for alcoholic fermentation, it is important to dilute the molasses properly, to acidify and to seed the musts. The addition of nutrient salts, and in particular of ammoniacal salts, may be advantageous in certain cases, as well as the elimination of part of the ashes and materials (organic acids, gums, etc.) hindering the development of the yeast. Molasses presenting large variations in the composition of the tests will be necessary in all cases to specify the best treatment to be applied.
syrups.
The syrups are concentrated cane juice, usually after lime defecation.
We use this raw material, on a small scale for that matter, to obtain rums of special quality, devoid of the “vesouté” taste of cane juice eaux-de-vie and possessing a finer bouquet than the rums of molasses. In addition, when sugar prices are low, it is more advantageous to produce syrups for export and as a raw material for the biochemical industries. This is what happened during the sugar crisis in Cuba, where it was made, under the name of high test molasses, large quantities of cane syrup for shipping on the United States.
Preparation.
Vesou cooked. — The cooked vesou is cane juice which is boiled rapidly for a few minutes, so as to have a final density of about 1.100. During boiling, the juice is carefully de-scummed. This product is still sometimes prepared in the French West Indies, in order to obtain a special rum intended solely for the consumption of a few amateurs.
Gros syrup. — Formerly known as gros sirop, the syrupy liquid, dark in color, from the dripping of raw sugars obtained by evaporation of cane juice.
This evaporation was done in a battery of 5 boilers, arranged in line on a solid masonry and heated by open fire. The set was called the crew or battery. The dimensions of these boilers, generally cast iron and hemispherical, decreased from the first to the last. The first, called the grand one, placed at the most hearty part, was used to perform the defecation of the vesou. The juice, after the addition of a small quantity of lime or ashes, and after the removal of the foam, was transferred by means of a bucket with a long handle into the second basin, called the clean one [la propre]. A certain amount of foam was still produced in it, which was rejected in the grand one. The syrup coming from the clean one received in the third boiler, or flambeau, an additional quantity of lime, if its limpidity left something to be desired, then was brought into the 4th boiler or sirop to a determined concentration. The cooking was finished in the 5th boiler, or battery. “The name battery,” says P. Labat, “is due to the fact that one is obliged to shake sharply with big skimmer, to prevent it from overflowing.”

FIG. – Battery of P. Labat and refreshers (Martinique nineteenth century).
In the various sugar-producing countries there were various modifications of the above model, known in the French Antilles as the battery of P. Labat, although it was earlier than the arrival of this monk in the West Indies.
In the equipment of Jamaica, for example, the hemispherical boilers, usually 3 in number, were preceded by a shallow, rectangular vessel placed at a higher level than the boilers and serving as a defecator.
Concretor, which has been used in some countries, consisted of a long plate (about 110 m), with partitions starting alternately on each opposite side. The plate was slightly inclined on its axis, so that the juice, received at the front part, flowed in a continuous stream on a small thickness, to the end of the apparatus. Under the tray was a furnace, but unlike the arrangement of other batteries, it was the part receiving the raw juice which was arranged above the hearth. The syrup arriving at the bottom of the apparatus entered a rotating cylinder, heated by the steam coming from the vesou. In some areas, the device was reduced to the tray without its accessories.
The mass-baked out of the battery was poured into very flat tanks (coolers), where it was allowed to cool, then in barrels opened from the top and whose lower bottom was pierced with holes. The barrels were placed on a slatted floor covering a tank, where the syrup from the dripping of the raw sugar flowed. The length of stay in the purgerie was 3-4 weeks.
When one wanted to obtain sucre terré, the cooked mass was introduced in forms, conical vases in unglazed earth, carrying at their extremity an orifice for the flow of the syrup. These forms were placed next to each other, the tip down. Once the syrup had been drained, the soil was put to the terrage, pouring on the sugar a layer of clayey earth, diluted in a thick slurry. The water that was in the clay slowly escaped and spread in the mass of sugar, bringing with it the viscous syrup that was there. The first layer of soil being entirely dry, it was replaced by a second and often by a third until the sugar was white enough.
The quantity of gros syrup obtained reached about 50% of the weight of the sugars, if these were simply subjected to the purging, and up to 60% if one carried out the terrage. According to Itier (1), in 1844, in Cayenne, for every 100 kg of sugar, 50 kg of molasses were obtained in the case of virgin cane, 33 kg with the first offspring and 20 kg with the second offspring.
(1) Ann. Marit. Col. 1844, t. 3. 626.
The manufacture of raw sugar by the crew system has been abandoned in the West Indies since the beginning of the century. The few remaining batteries produce only syrup for culinary consumption. However, in South America, and particularly in the Brazilian countryside, there are still facilities for the preparation of brown sugar.
Battery syrup. — The battery syrup was formerly obtained by the crews. The juice was treated in the same way as for the manufacture of sugar. However the concentration was pushed only to around 65-70° Brix, which allowed removing the last basin used for cooking syrup. [only used 4 of the 5 basins]
In some islands of the West Indies (Barbados), as well as in the United States (Louisiana, Florida), there are still a number of facilities of variable importance which prepare syrups by evaporation of cane juice in open boilers. However, the product obtained is generally intended for table consumption. On the other hand, the old “crews”, which required considerable fuel expenditure, have been modernized. Cylindrical or rectangular evaporators constructed of copper or galvanized sheet are usually heated by means of steam coils. Continuous evaporators consisting of a long tray with transverse partitions to allow the juice to flow slowly by gravity from the inlet to the outlet of the apparatus, are widespread in the United States (Walton, Ventre and McCalip (2).
(2) Farm production of sugarcane sirup Farmers Bull. no 1874, 1941.
In the distilleries of a certain importance producing rum of syrup, as found in the French West Indies, the concentration of the vesou is done in vacuum apparatus with multiple effect analogous in number and dimensions to those of the sugar factories. The concentration of the syrup can vary from 55 to 75 ° Brix.
The juice is usually defecated with lime (3), as follows. It is first added, in heaters equipped with a vertical vane agitator, the amount of milk of lime necessary to reach the point of neutrality. It is then brought to the temperature of 80-90° in a tubular heater, then to that of boiling in open defecation boilers, equipped with a coil fed with live steam and generally 3 in number to allow continuous work.
(3) Sulphitation of the juice, sometimes practiced in Louisiana for the preparation of table syrup, is not used in the manufacture of distillery syrup.
The clear juices from the defecators are filtered with Philippe filter or on very fine mesh flat filters before being sent to the evaporation chambers. As for the turbid juices, they are passed to filter presses, then sent to clear juice filters, to be mixed with the previous ones.
Liming juice is not always practiced. In this case the clear liquid from the defecators is sent as such to evaporation, without passage to the filter Philippe: the turbid juices are treated with filter press. In other cases, in order to prevent the crystallization of the sugar, which occurs when the syrup reaches a high concentration, a certain quantity of sulfuric acid or tartaric acid (1 gr per kg of sugar) is added, to determine the inversion of a part of the sucrose.
Invert syrup. — The product known as high test molasses is prepared in Cuba by partially inverting lime-defecated syrups which are neutralized and then concentrated to the normal density of the molasses (82-84° Brix).
Inverting is usually done with sulfuric acid (1 to 2.5 l per 1,000 l of syrup) at temperatures varying between 75 and 90 °. This procedure has several disadvantages. The rise in temperature determines the destruction of a certain amount of sugar (levulose). On the other hand, if the neutralization precedes the concentration, abundant encrustations are formed in the evaporators, and if it follows it is very difficult to obtain a suitable mixture of the lime and the syrup.
Inversion by means of invertase avoids these disadvantages. In the Guerrero process, the juice from the last mills, which, because of its high nitrogen content, is suitable for yeast culture, is pasteurized, cooled and seeded with a special yeast, which contains about 10 times more invertase as ordinary baker’s yeast. The yeast obtained after passing through a contractive separator is used to seed continuously the flow of syrup from the evaporators. 4 volumes of yeast suspension (concentration 12% of the original yeast milk) are used per 100 volumes of syrup at 53° Brix. After 10 hours, 65% of the sucrose of the syrup is inverted.
Composition.
The composition of syrups is even more variable than that of molasses, because of the variety of preparation techniques.
Gros syrups, or molasses of brown sugar, which present little more than a historical interest today, differed from the present sugar molasses by their greater richness in crystallizable sugar and their relative poverty in reducing sugars and minerals. Here are some analyzes of gros syrups produced in Martinique and Louisiana.

Hereinafter the composition of battery syrups of various origins:

As for inverted syrups from Cuba, they contain 18 to 30% sucrose (average 23%) and 30 to 60% reducing sugars (average 52%). It should be noted, by way of comparison, that blackstrap molasses from the same country average 35% sucrose and 20% reducers.
As fermentation raw material, the syrups are characterized by their lack of nitrogen and mineral matter. The musts prepared from these products require, in order to ferment properly, to be supplemented with ammonia nitrogen and certain nutrients (phosphates), sometimes to be acidified (syrups defecated with lime). Syrups, with the exception of gros syrups, are particularly poor in ferments, as shown in the following table which indicates the number of microorganisms existing per cc. or by gr. of material at different stages of sugar production (Owen Church, Kopeloff):

Syrup must therefore be mounted with a pied de cuve. If left to spontaneous seeding, the start of fermentation will be very slow.
Foams, washings, etc…
In addition to molasses, the sugar factories produce various by-products (feces of defecation, washings), which are generally mixed with the preceding one, but which in some cases can be used for the composition of special musts. On the other hand, in order to produce certain types of rum, sugary juices are sometimes subjected to treatments, which profoundly modify the quality. This is particularly the case in the production of Jamaican rum, where the “acid” and “aroma” added to the musts influence the nature of the fermentation and the bouquet of the eau-de-vie obtained.
Scums. [Hard to say if écumes should be translated at scums or foams.]
This name refers to the mousses and deposits that occur during the defecation of cane juice.
Scums were formerly of great importance as raw material of rhummerie, at the time of the “sugar houses”, when one made brown sugar by the process of the crews. After the establishment of the central factories, in some cases (in Jamaica and sometimes in the French West Indies), the skimmings obtained in the open defecation boilers (1) were sent to the distillery. But most often they are subjected to a filtration to recover the sweet juice, which goes into the manufacture. At present, factories using the Petree-Dorr treatment process, which tends to become widespread in the West Indies, no longer produce defecation scum.
(1) When defecating juice is brought to a high temperature in the heaters and added hot milk of lime, which usually takes place in modern sugar factories, the impurities fall for the most part at the bottom of the boilers of defecation, constituting “sludge” rather than “scums” Formerly on the contrary, when the juice was not de-aerated by a preheating, but after cold liming it was gradually carried in the defecator around the boiling, most of the impurities rose to the surface.
The scums, to which was added the washing water of the boilers and the various residues of the sugar mill, were received in a special vat, where they were left to decant, the clear liquid being alone used for the composition of the musts. The deposit was discarded, sometimes after washing with water, so as to recover the remaining sugar.
“Scums, out of the sugar factory,” Ducoeurjoly writes (1802), “will be deposited in a cistern, or in a vase large enough to contain all those removed from the sugar boilers, in 48 consecutive hours of work. They should be used only after this space of time, which is necessary to establish a beginning of fermentation, which drives out and rejects on the surface all the refuse with which they are charged; by this means we greatly diminish the daily care we give ourselves to skim the grappes. We must not hurry to use the foam, it is essential to wait until they are well cleaned and cleansed, by their own fermentation… The materials that are removed, by scumming the vase where the foam is deposited, are excellent food for the horses, the oxen and the mules, they fatten them, but we must not give them too hot.”
Wray wrote on his side: “Scums comprising all the substances separated from the cane juices during the operations of the defecation and the evaporation, we thus have the thick crust of scum formed on the surface of the liquid in the clarifiers, the scum of the precipitators and evaporators and the deposits formed at the bottom of these two series of vases. A portion of sweet liquor is mixed with the different scums and deposits, plus a large amount of water that was used to clean the various vases and pipes. Later, each time the liquid is removed from the evaporators, as happens every Saturday night, a certain amount of what is called “soft liquor”, resulting from washing the boilers, etc., will join the scum. All this flows together in the distillery, mixes in the container with the scum, and takes the name of “residues”. The liquid part of these residues, when clarified, is withdrawn and takes the name of skimmings proper…”
“When the residues of sugar manufacture flow to the distillery, they are received in what is called the scum container, which usually holds 300 and 400 gallons. This vase is always lined with rolled lead or very thin copper; it is equipped with a tap to extract its contents. A container being full, the liquid is allowed time to clear. The thinned liquor, then properly named skimmings, is drawn into the tank to the mixture if there is one, or in the opposite case it goes directly into the fermentation tank.”
(1) When defecating juice is brought to a high temperature in the heaters and added hot milk of lime, which usually takes place in modern sugar factories, the impurities fall for the most part at the bottom of the boilers of defecation, constituting “sludge” rather than “scums” Formerly on the contrary, when the juice was not deaerated by a preheating, but after cold liming it was gradually carried in the defecator around the boiling, most of the impurities rose to the surface.
“All remaining impurities in the container are emptied into another container immediately below the first, where they are mixed with 100 or 200 gallons of water (hot if available) and well mixed, long enough to separate all the sugar. This work must continue until the sugar has been washed well, the liquid mass then remains some time at rest to clear again. If there is reason to fear fermentation or acidity, in the first case, a little sulfuric acid is added, by burning one or two sulphured matches, and in the second, a certain quantity of lime. The clarified liquid can be poured into the fermentation tank to help prepare the liquid to be distilled. I know that the washing of the deposits is rarely used, but it certainly should be, otherwise what these deposits contain of sweet matter would be discarded.”
Scums usually entered a certain proportion in the composition of musts: Wray indicates that of 2 skimmings for every 1 of molasses, as the best and most economical. Rarely, they were used alone. Rich in microorganisms and nitrogenous matter, they contain a relatively small amount of sugar, they fermented very quickly and easily underwent acetic acidification. Mixed with molasses, they facilitated the start of fermentation. The musts obtained from the scums alone gave a rum with pronounced taste and often of inferior quality (burnt taste).
Boname (1) gives the following composition for raw (not decanted) scums:
(1) Culture de la canne & la Guadeloupe, Paris, 1888.

The ashes, relatively rich in phosphoric acid, contain, on average, according to the same author:

Allan indicates as composition clarified scums used in Jamaica in the composition of the mouts:

The acids, total and volatile, are evaluated in sulfuric acid as in the analyzes of the same author which we reproduce later.

In Jamaica, the scums are allowed to sour for a few days before using them. For the production of high-flavored rums, they are sent in tanks half-filled with bagasse and cane straw, where they stay for 4 to 6 days. The acidity of ripe froths is already higher than it is proposed to get a more full-bodied rum.
This acidity is mainly due to the presence of fixed acids, of which lactic acid predominates and which represent 75 to 80% of the total acids. Since fresh scums have a neutral reaction, the acids originate during the microbial fermentations that develop during the conservation of the scums. Ashby has been able to isolate from them many bacteria, among which he has almost always found a lactic acid bacterium, which forms rounded gelatinous masses in the liquid.
Scums obtained in the past had a higher saccharin content than those indicated above. It was usually considered that 60 liters of foam had the same sugar content as 10 liters of molasses. In some cases, however, it took up to 1 hl. of scums to give the same amount of rum as 10 liters of molasses.
Washings.
Formerly referred to as washings stricto sensu, the product from the washing trays containing molasses. The latter being badly exhausted, a layer, several inches thick, of a blackish sugar, which was dissolved by means of hot water, was deposited at the bottom of the vats. The liquid obtained, marking about 32° Brix, was often used alone in the preparation of musts. It went into fermentation very quickly and gave a light rum with a finer bouquet than the molasses rum.
In a broader sense, the liquids from the washing of the various containers which have contained sweet substances (juice and syrup trays, defecation boilers, etc.) are called washings. These liquids are normally sent to the distillery, where they are combined with the foam or mixed, during the composition of musts, with the other raw materials.
Acid and aroma.
In the manufacture of grand-arôme Jamaican rum (german rum type) cane juice is used which has been the subject of a preliminary treatment to develop certain aromatic principles (acids, esters).
The “acid” (acid) is obtained by subjecting the juice, previously defecated with heat and adding vinasse and sometimes a little scum, to the alcoholic fermentation. When this is almost complete, the liquid is directed into tanks half filled with bagasse and cane straw, where it is allowed to acidify. From time to time deposits of fermented musts are also sent from these tanks. From the first tank, the liquid is transferred in a second, then in a third. At the end of the latter the “acid” is good to be used in the composition of the musts. It then has the smell of sour beer.
The “arôme” (flavour) is prepared by fermenting cane juice with vinasse from the previous year (clear part), in tanks containing bagasse. It also usually contains a certain amount of the liquid from the muck hole, which is sent the deposits of vinasse and small waters, appliance washing water, bagasse and other residues of the plant.
We give below, according to Allan, the composition of the products obtained. These are particularly rich in fixed acids.

The bottoms, formed mainly by yeast cells mixed with loose bagasse, seem to play a particularly important role in the “maturation” of the acid and the aroma (1). Those obtained in the manufacture of german rum have, according to Allan, as composition:
(1) According to Cousins, esters and high-molecular-weight acids, which play an important role in the formation of the bouquet of high-flavored Jamaica rums, would come from the bacterial decomposition of dead yeast cells found in fermentation muck and vinasse muck.

Boname gives the following analysis of 3 bottoms from the fermentation of molasses in Mauritius (gr per liter):

Vinasse. [Dunder]
Vinasse, more commonly known as vidange [emptying or drainage] in the French West Indies and dunder in English countries, is the residue of the distillation of fermented musts.
Formerly, in the French West Indies, the vinasse was received at its output from distillatory apparatus in large surface masonry ponds, in order to facilitate cooling. In the important distilleries, there were the continuation of each other several basins, that the liquid crossed successively, so as to leave the last sufficiently cooled for the use. This cooling in the open air has the disadvantage of allowing the invasion of the vinasse by all kinds of bacteria and harmful germs. Also, today, in somewhat large installations, tubular refrigerants are usually used, which bring the temperature of the liquid to 30-35°. When water is lacking, cascade refrigerants are also frequently used. These consist of several stages (5 to 10) of fascines or slats, on which flows the vinasse, distributed at the top of the refrigerant by wooden gutters pierced with holes.
From the point of view of composition and properties, it is advisable to distinguish the vinasse from vesou and the molasses vinasse.
The first is a yellowish, sparse liquid, usually between 1.010 and 1.020 density (3 to 50 Brix). Its acidity is quite variable, depending on the purity of the fermentations. It is generally between 3 and 5 gr. In the beginning of the century, when the fermentations were longer and less pure, the acidity of the vesou vinasse reached 6 to 7 grammes per liter (Pairault) in the French West Indies. Dry matter, usually between 30 and 50 gr. per liter, contains on average 4 gr. ash rich in phosphoric acid and potash.
Molasses vinasse is dark brown in color and has a much higher density than vesou: 1.040 to 1.075 (about 10 to 19° Brix), but sometimes as high as 1.090 when it comes from concentrated musts used in manufacturing grand arôme rums. Its average acidity, in the case of pure fermentations, is 4 to 6 gr. per liter. But it reaches up to 30 gr. per liter, for the vinasse of grand arôme rum. Between these extremes, all the intermediaries can exist. Pairault, at the beginning of the century, indicated as average acidity of vinasses from molasses of the French West Indies 10-11 gr. per liter. The volatile acidity (formed mainly by formic acid) varies between 10 and 40% of the total acidity. Dry matter (80 to 200 grams per liter) comprises 20 to 50 gr. per liter of ash rich in potash; 3 to 9 gr. nitrogenous material; varying quantities, but generally low (2 to 4 grams per liter per liter), sugars having escaped fermentation and unfermentable reducers..
Boname (1) gives the following percentage composition for molasses vinasse from Mauritius:
(1) Ann. Sc. Agron. (2) II 215, 1896.

The mineral substances were composed as follows (in grams per liter):

According to Pairault, the proportion of nitrogenous matter is rather variable. When sulphate of ammonia is added to musts, this salt, if it is used in excess, is found partly in the vinasse and increases the total nitrogen content. On the other hand, during storage, there is a deposit of nitrogen-rich materials, so that the results are substantially different depending on whether one operates with the troubled or clarified vinasse. The above author has found, for various vinasse from Martinique, a content of 7 to 9 gr. total nitrogen content per liter. The nitrogenous materials were distributed as follows in a clear and slightly old sample of vinasse (in grams per 1):

Nelson and Greenleaf found as a percentage basis of the dry matter of a cane molasses vinasse from a Baltimore distillery:

In addition to acetic and formic acids, they identified the following fixed acids:

Tricarballylic acid, CO2H – CH2 – CH (CO2H) – CH2 – CO2H, comes from the reduction of aconitic acid during fermentation. Succinic acid and most of lactic acid are also products of molasses fermentation.
Allan indicates as composition of vinasses used in Jamaica in the manufacture of rums of various types:

In the manufacture of certain types of rum, vinasse is conserved from one campaign to the next. Some distillers, in order to prevent the alteration of the vats when they are empty and dry, also fill them with vinasse: the latter being acid does not putrefy and the vats are well preserved.
After a while, the vinasse is covered with a thick veil of mold. which prevents access to the air, and clarifies itself, constituting a yellowish liquid of bitter taste, often used in the preparation of musts for grand arôme rum. [Percival] Greg found that it contained a very aromatic acid, which smells of ethyl acetate and lemon oil. This body, which the author named fruity acid and found in Jamaican rum, appears only after the vinasse has already undergone acetic fermentation and the formed acetic acid has disappeared in part. It can be extracted with petroleum ether.
Finally, vinasse, if not used immediately, contains many microorganisms, including yeasts of the type Schizosaccharomyces (Pairault) and bacteria (Kayser).
Vinasse plays an important role in the fermentation of musts and the production of the bouquet of rums. It brings appreciable quantities of nitrogen and mineral matter, foods of the yeast, and especially of acids, which promote the esterification, protect the yeast against the competition of the microbes and ensure the predominance of certain races of ferments (1). The regulating role of vinasse has been known for a long time. Wray already wrote in 1848: “It increases the density of the liquid, prevents the excessive violence of the fermentation, during which so much alcohol is lost, and holds the liquor at a comparatively low temperature, in a slow and moderate internal working state.”
(1) Fixed acids, on the other hand, would play a useful role in helping to fix certain basic products made by bacteria which, by passing through the distillate, would be detrimental to the quality of the rum (Allan).
It also intervenes, both by the aromatic principles that it brings and the influence it exerts on the fermental flora, in the formation of the bouquet. The rums obtained by using high proportions of vinasse are less fine, but more full-bodied and more aromatic than the others.
Finally, as there is sometimes a certain amount of fermentable sugars as a result of defective fermentations or even alcohol, the return of the vinasse in the manufacture can lead to fairly significant increases in yield.
In the beginning of the rhum industry, it was even avoided to push the alcohol exhaustion of the vinasses too far. “In order for them to have all the qualities demanded of them,” writes Ducrurjoly (1802), “the distillation of the little water must not be too much, for fear that it will strip them entirely of all their spirit parts, and do not impoverish them to the point of being no longer fit to be employed. ”
The distillation was stopped when the alcoholic strength of the small waters [tails] fell around 34° G.L.
Dilution water.
The chemical and bacteriological composition of the water used in the preparation of musts influences the course of fermentation and the quality of the product obtained.
The mineral substances contained in the water, particularly the salts of soda, magnesia, lime and iron, increase the value of the nonsugar ratio: sugars, which, in the case of molasses musts, principally may be detrimental to the development and the diastatic activity of yeast. It is especially in the last stages of fermentation that the harmful action of these salts is felt. The typical gear of the fermentation can be more or less disturbed, with formation of secondary products, which modify the bouquet of the eau-de-vie. In addition, during the distillation, they cause deposits on the trays and in the supply lines of the columns. reducing the performance of these and forcing frequent stops to clean the devices.
In principle, waters heavily loaded with mineral matter (calcareous, magnesian, ferruginous waters) are to be rejected. The same is true of seawater, which is however sometimes used and may in certain cases (musts low in mineral salts) (2) have a useful effect on fermentation (see Chapter V.).
(2) The use of seawater is reported by ancient authors. Ducourjoly especially recommends it in the case of musts prepared without vinasse and scums, at the rate of 25 of sea water per 100 of ordinary water.
Water contaminated with organic matter, polluted by manure pits or in any other way is particularly harmful, as a result of the presence of decomposing materials (ammoniacal combinations, nitrites, nitrates, chlorine compounds, etc.) toxic to yeast and which can pass partly in the distillate.
The bacteria contained in the water, especially in those of pools and groundwater polluted with organic matter, cause secondary fermentations and give rise to volatile products of unpleasant smell or taste.
The water intended for the preparation of musts must therefore be as pure as possible. Unfortunately, the industrialists are often forced to work with water of inferior quality and even frankly bad. In the French West Indies in particular, where the rum campaign usually coincides with the dry season, distillers frequently draw in the rivers at low water level, soiled by local residents, who come to wash their clothes.
Bettinger, however, did not find any significant differences in yield using, for the composition of the must, both ordinary water and soapy water from the creek.
It is interesting in some cases to correct the water intended for manufacturing. Those with high levels of nitrites, nitrates, chlorides or organic matter are not likely to be effectively treated. On the other hand, waters which contain carbonate of soda, potash, lime or magnesia are immediately improved by the addition of a quantity of dilute sulfuric acid, calculated so as to neutralize the alkalinity of these carbonates. The ferruginous waters, which weaken the yeast, can be treated by aerating them strongly by passing over beds of coarse gravel: the iron, which is mainly in the form of ferrous carbonate, precipitates in the state of oxide, which is then separated by sand filtration. Finally, fine sand filtration is suitable for murky waters.
Boiling is an effective method for ensuring the bacteriological purity of water, but it is too expensive to be practical. The actual treatment method applicable in the fermentation industries is sterilization by ultraviolet rays or, better, by ozone.
CHAPTER III
LES FERMENTATIONS EN DISTILLERIE (1)
(1) Van Lear – La chimie des fermentations. Paris 1935.
The sweet musts left to themselves are the seat of complex phenomena. Parallel to or after the alcoholic fermentation, secondary fermentations (acetic, butyric, lactic, putrid, etc.) develop. These always have the effect of reducing the yield of alcohol. So they are considered undesirable and usually do their best to avoid them, especially in the distillery of industrial alcohol. In certain cases, however, when one wishes to obtain eaux-de-vie with pronounced bouquet, it is allowed and even promotes secondary fermentations generating aromatic principles.
In the rhummerie, the importance of the latter is most variable. In the production of light rums, pure yeasts and sterilized or antiseptic media are used to achieve pure alcoholic fermentations. On the other hand, microbial agents, and mainly butyric bacteria, play a very important role in the production of Jamaica’s very strong rums. Between these extremes, we find all the intermediate fermentation complexes.
Alcoholic fermentation.
It is only at the end of the eighteenth century that the first precise information on alcoholic fermentation dates back. Lavoisier, applying his methods of analysis, showed that sugar was splitting into alcohol and carbon dioxide. Few years later, Gay-Lussac translated these results by the general formula:
C6H1206 = 2 C2H6O+ 2 CO2
Lavoisier had only considered the chemical aspect of the phenomenon, without expressing any hypothesis as to the nature or the mode of action of the ferment indispensable to effect the transformation.
Although this ferment was observed under the microscope and described by Leuwenhoek in 1680, its nature as a living being was established only around 1825, by Turpin and Cagnard-Latour in France, Schwann and Kutzing in Germany, Pasteur showed, in 1859, that fermentation was a correlative process of yeast life, contrary to Liebig’s theory that the decomposition of sugar was due to a molecular shattering caused by the ferment.
While recognizing the living nature of yeast, various scientists, including Claude Bernard and Berthelot, believed that its action on sugar was due to a diastase separable from the cell. The Buchner brothers were able to isolate, in 1897, the alcoholic zymase. Harden and Young have shown that it is formed by a mixture of two substances, inactive separately and recovering their activity when they are mixed: the one colloidal and losing its fermentative properties by heating, apozymase or ferment itself; the other dialysable and thermostable, the cozymase or coferment of the zymase. [SOS something seems amiss in the last lines here.]
Mechanism of fermentation.
The classic Gay-Lussac formula gives only a very general idea of the complex phenomenon of alcoholic fermentation. It does not take into account intermediate bodies or secondary products (succinic acid, glycerin, etc.) which originate.
It is now generally agreed that carbonic acid and alcohol derive from a 3-carbon body, which itself derives from the breaking up of the hexose molecule.
Bayer (1870), and after him Wohl, regarded as an intermediate body lactic acid, which is often found among the products of alcoholic fermentation. In a first phase, the zymase would transform glucose into lactic acid, while in a second phase another diastase), lactacidase, break down this acid into ethyl alcohol and carbon dioxide. In fact, if lactic acid could be obtained by reacting yeast juice with glucose solutions (Oppenheimer), it was not possible to convert the lactic acid into alcohol by live yeast. So this theory is abandoned today.
We then thought of dioxyacetone CH2OH – CO – CH2OH (Wohl, Buchner, Fernbach).
This could be obtained chemically and biologically at the expense of hexoses and gives alcohol by fermentation. Unfortunately, it has so far been tried in vain to produce it by yeast, so that its formation during the alcoholic fermentation remains purely hypothetical.
According to other authors, notably Neuberg, the first step in the cleavage of the hexose molecule would result in hydrated methylglyoxal (or pyruvic aldehyde). This one, by simple dehydrogenation, would give the pyruvic acid, which would decompose, under the action of a carboxylase, in acetaldehyde and carbon dioxide. Finally, the acetaldehyde would provide, by reduction, the ethyl alcohol. These successive transformations are summarized by the following equations (Neuberg scheme):

The formation of pyruvic acid during the alcoholic fermentation was demonstrated by Fernbach and Schoen, who were able to obtain it by adding chalk to the liquid, so as to prevent any acidification of the medium. This acid decomposes very easily. under the influence of yeast to give acetaldehyde and carbon dioxide.
The acetaldehyde has also been obtained directly in the fermentations carried out by the living yeast: by adding alkali sulphites to the must, it is easy to bind (Müller-Thurgau, Neuberg). Finally acetaldehyde can be reduced by yeast, giving alcohol (Kostytschew).
There is therefore a growing tendency to admit that pyruvic acid and acetaldehyde are indeed intermediate products between sugar and alcohol. With regard to pyruvic aldehyde, the question is more debated. The presence of methylglyoxal has only been observed in a small number of cases, especially in bacterial fermentations (Fernbach, Aubel). It is only in the special conditions, in the absence of coferment, that Neuberg observed his formation from glucose, under the action of yeast extracts. In addition, methylglyoxal is not fermentable by yeast (1). It is therefore considered today, with Meyerhoff, that this aldehyde is an accidental product of fermentation, arising purely by chemical means, and that the precursor of pyruvic acid is probably glyceric acid.
(1) This can not, however, be considered as absolute proof. It has been pointed out, in fact, that the form in which methylglyoxal takes place during the metabolism of sugar may be much more labile than in the state in which we handle it. In addition, the product, which is normally used as it is formed without ever accumulating in the medium, risks, if introduced in bulk, to distort the mechanism of the transformations. These arguments, which also apply to other intermediate products, have often been used by the authors to explain the difficulties of fermenting these substances in vitro.
It is generally admitted, following the work of Harden and Young, that phosphoric acid combines with sugar, in the presence of zymase, to give a phosphorus-containing organic compound, hexose-diphosphate or fructose-diphosphate, which is attributed to following formula:

According to Meyerhoff (2). this body would split, under the action of a diastase, the zymohexase, in 2 molecules of a triose-monophosphate, the phosphoglycérique aldehyde or phosphoglycéral:
(2) Ann. Inst. Pasteur LIII, 221, 1934.
![]()
Two phases should be considered, differing in their speed: the incubation or induction phase, which is slower, and the actual fermentation or stationary phase. The first would involve the transformation of the phosphoglyceric aldehyde, by oxidation-reduction, into the corresponding acid and alcohol (phosphoglycerolic acid and phosphoglycerol):

Phosphoglycerol (or phosphoglycerol alcohol) stays away from the fermentation cycle (saponified, it gives rise to glycerin), while phosphoglyceric acid breaks down into pyruvic acid and phosphoric acid:
![]()
Pyruvic acid gives, by decarboxylation, acetaldehyde:
![]()
We then go to the stationary phase where the speed of the phenomenon accelerates: the acetaldehyde reacts on the phosphoglyceric aldehyde, to form by oxidation-reduction, alcohol and phospoglycérique acid:

The phosphoglyceric acid decomposes, according to reactions 3 and 4, to release carbon dioxide, phosphoric acid and acetaldehyde which enters the reaction.
In support of Meyerhoff’s theory, the following facts are invoked. By the use of various paralyzers of fermentation, it was possible to highlight the above transformations. Thus, monoiodoacetic acid prevents the decomposition of the phosphoglyceric aldehyde and stops the transformation when the reaction (1) has been completed. Fluoride hinders reaction (3): a mixture of phosphoglycerol and phosphoglyceric acid is obtained in its presence. Finally, by adding alkaline sulphites to the must, acid pyruvic acid and glycerine are formed. It has also been observed that the addition of acetaldehyde to a sweet must completely suppresses the period of induction of fermentation.
It remains, however, that hexose diphosphate, if it has been possible to obtain it by action of yeast extracts on fermentable sugars, has not yet been isolated from the musts in fermentation, and that, if introduced in the musts, it is not transformed by the yeast. It is then reduced to suppose that the diphosphoric ether is used as it is produced, and that the one which is added to the liquid does not penetrate inside the yeast cells.
It has also been observed that the various phosphoric ethers above (hexose-diphosphate, phosphoglycerol, phosphoglycerol and phosphoglyceric acid) ferment less easily than sugars in the presence of yeast extracts. They may originate in a more labile and fermentable form than that which we know or intervene in the reactions of activators we do not know (1).
(1) In the presence of traces of arseniate, for example, the fermentation of phosphoric intermediates is greatly accelerated.
A nitrogenous base, adenosine, appears to play, in the form of adenylic acid (adenine monosphosphoriboside) and adenosine-phosphoric acid (adenine triphosphoriboside), which is always found in yeast, an essential role in the phophorylation of sugars.
Adenosine triphosphoric would be the lessor of phosphoric acid for the etherification of sugars, and phosphoric acid, by regenerating the acid adenine-triphosphoric acid. This appears then as the catalytic factor of the phosphorylation and would constitute, according to some authors, the cozymasé.
Secondary products of fermentation.
During the alcoholic fermentation, ethyl alcohol and carbon dioxide are also formed, various secondary products which come, some of the “vegetable function” of the yeast (higher alcohols, succinic acid, etc.), others of the “zymatic function” (glycerine, aldehyde acetic, etc.). The nature and the proportion of these products vary within wide limits, according to the conditions of the medium, the yeast being “a plastic organism, whose physiological properties can undergo important modifications under the influence of various factors” (Fernbach).
Glycerin. – In his “Memoir on Alcoholic Fermentation” (1859), Pasteur reported for the first time that glycerine or glycerol, CH2OH – CHOH – CH2OH, was a constant product of fermentation.
Laborde (2) observed that the quantity of glycerine, relative to 100 g of sugar disappeared, varied within wide limits: 2.50 to 7.75 g (2.5 to 3.5 on average). He showed that all the causes of weakening of the yeast contributed to increase the production of glycerine: long duration of the fermentation, richness of sugar, abundant multiplication of the yeast by aeration, etc. The nature of sugar and the yeast race also have an influence.
(2) C. R. CXXIX, 344, 1899, Ann. Brass. Dist. II, 473.
Neuberg, then Connstein and Ludecke (3) have shown that, by the addition of sodium sulphite to the must, there is an increase in the yield of glycerine, up to 36% of the sugar. This process was used industrially in Germany, during the 1914-18 war, to obtain the glycerin necessary for the manufacture of explosives.
(3) Ber. Deut. Chem. Ges. LII, 1385, 1919.
Another method, due to Eoff, Linder and Beyer (4), is to ferment the sugar in the presence of baking soda (or other alkalis), which is added to the liquid after the start of fermentation, gradually and in small portions up to a dose of 5%. 20 to 25% of the sugars in the must are converted to glycerin, the balance providing alcohol, acetic acid and carbon dioxide, with small amounts of various other poorly identified products.
(4) Ind. Eng. Chem. XI, 843-45, 1919.
Glycerine is generally considered a “side product” of sugar processing into alcohol. When the train of reactions is deflected, preventing the medium from being acidified or adding sulfite, the chemical specificity of the yeast, which becomes a glyceric ferment, is modified.
We would have in the first case:
![]()
and in the second case:

In the Neuberg scheme, the pyruvic acid or the acetaldehyde being fixed by the alkalis or the sulphite, the hydrogen coming from the reduction of the methyglyoxal would react with one of the molecules of the latter, which it would transform into glycerin.
According to Meyerhoff’s theory, the glycerine would come from the saponification of phosphoglycerol, the bisulphite intervening to block the ethyl aldehyde [acetaldehyde by another name] as it is formed and prevent its reaction with phosphoglycerol aldehyde.
It is explained that a certain amount of glycerin always occurs in the alcoholic and alcoholic fermentation in the glycerol fermentation, because the yeast never behaves like a pure alcoholic ferment or a pure glyceric ferment. The two transformations would develop side by side, in varying proportions according to the conditions of the environment.
In ordinary alcoholic fermentations, glycerin is formed on the whole at the end of the fermentation, when only a small quantity of sugar remains, and the nutrition conditions are unfavorable to the yeast.
Aldehydes – Acetic aldehyde, or acetaldehyde, CH3-COH, has long been reported among the products of alcoholic fermentation (Roeser, 1893). It usually exists only in very small quantities. However, in wines, it was possible to isolate up to 860 mgr of aldehyde per 100 grs of sugar consumed.
It is generally accepted that it constitutes an intermediate body in the transformation of sugar into alcohol. Muller-Thurgau and Osterwalder have shown that sulphurous acid and sulphites, added to the musts of fermentation, combine with the aldehyde, which is thus fixed and can no longer be converted into alcohol. In this way 18-24% of the sugar could be obtained in the form of aldehyde. Liquids fermented in the presence of SO2 would thus normally contain a larger amount of acetic aldehyde.
Acetaldehyde can also be produced by oxidation of ethyl alcohol. Kayser and Demolon have observed that the residence of wines on lees, in broad contact with the air, favored the production of aldehyde and that the living yeast was the essential agent of this phenomenon. Trillat and Sauton (1) showed that yeasts were likely to produce significant amounts of acetaldehyde at the expense of alcohol within a few hours: up to 2,500 mg per 100 alcohol at 100° after 6 hours. The aldehyde formed disappears little by little, if the contact with the yeast is prolonged.
(1) C. R. CXLVI, 998, 1908.
Finally, the aldehydeification of the alcohol can take place by purely chemical means, especially during the distillation and when the eaux-de-vie are treated with porous (charcoal filtration) or oxidizing (oxygenated water, permanganate, etc.).
Furfurol, or pyromucic aldehyde, mainly arises during distillation, by prolonged boiling or roasting of certain carbohydrates. Kayser and Demolon, however, believe that the yeast itself must intervene in its production. These authors observed that the proportion of furfurol underwent variations according to the nitrogen diet: it was minimal in the case of yeasts receiving sulphate of ammonia and instead increased substantially in the presence of leucine. It is not impossible, therefore, that furfurol constitutes an excretion product, linked to nitrogen disassimilation.
Higher Alcohols. – It is only towards the end of XIX century, following the works of Pasteur, Wurtz, Windisch etc … that the composition of the oil of fusel, or “fusel oil”, whose presence had been known since Scheele (1785), could be specified. This consists of a mixture of higher alcohols, qualitatively and quantitatively variable depending on the origin of the brandy, but among which amyl and butyl alcohols predominate.
Today, these higher alcohols have two different origins. Some (normal butyl alcohol) come from the attack of carbohydrates by bacteria, while others (amyl alcohol, isobutyl alcohol, etc.) result from the degradation of amino acids by yeast. The high levels of higher alcohols in alcoholic liquids almost always result from bacterial interventions.
F. Ehrlich (1) showed, in 1905, that the higher alcohols which originate during alcoholic fermentation are the by-products of the nitrogen nutrition of the yeast. When it has at its disposal, as nitrogenous food, only amino acids, it is to the amino group of these latter that it borrows the nitrogen which is necessary for the construction of its cells. Amino acids first undergo de-amination (loss of NH3, then decarboxylation (loss of CO2), ultimately to provide an alcohol containing one carbon atom less than the amino acid that gave rise to it. Thus isobutyl alcohol would originate from valine, isoamyl alcohol from leucine:
(1) Z. Ver. Rubenzuckr. Ind. 1905, 539 : Ber. Deut. Chem. Ges. XL, 1027, 1907

Thorme (2), adding different amino acids to a nutrient medium, was able to recover, after fermentation by yeast, the corresponding higher alcohols, in amounts closely proportional to those of the amino acids employed.
(2) J. Inst. Brewing XLII. 13, 1939.
Ehrlich assumed that the production of higher alcohols at the expense of amino acids was the result of simple hydrolysis and could be represented by the general formula:
![]()
The reaction would actually be much more complex, according to Neubauer and Fromherz (3), who consider that the process of amino acid degradation actually corresponds to an oxidation-reduction, and proceeds as follows:
(3) Z. Physiol. Chem. LXX, 326, 1910.
1°) oxidation of the amino acid and production of a hydrate of the corresponding amino acid:
![]()
2°) proper de-amination, giving rise to the ketonic acid of the same series:
![]()
3°) decarboxylation and production of an aldehyde having one less carbon atom than the amino acid:
![]()
4°) finally reduction and production of the higher alcohol:
![]()
This process has a striking analogy with that of sugar fermentation: the ketonic acid obtained in the second stage of the transformation is a homologue of pyruvic acid and the last two stages are the same in the one and the other case.
Ammonia from the disaggregation of amino acids can never be found as such in musts. It must therefore be converted into insoluble protein materials, forming part of the yeast cells.
Ehrlich observed that in ordinary fermentations of pure sugar, the amount of fusel oil produced ranged from 0.4 to 0.7% of the alcohol formed. These figures are close to those achieved in industrial practice. Thus Martraire (1) was able to obtain, with apple musts, an average production of 0.512% fusel oil to alcohol in the year 1928 and 0.520% in 1929. Houssiau (2), in 1932 and 1936, in the case of musts of beet and cane molasses, an average annual yield of 0.306 to 4 233% of crude alcohol and 0.191 to 0.265% of sugar was observed (3).
(1) in Pérard. — Le rendement en distillerie [Distillery yield]. C. R. 5. Cong. Int. des Ind. Agr. 802 – 832, 1937.
(2) C. R. 5. Cong. Int. des Ind. Agr. 864, 1937.
(3) The figures given, which correspond to the fusel oil obtained industrially, are somewhat inferior to reality, because part of the higher alcohols (10%) is removed during the distillation with the means tail tastes.
The production of fusel-oil is higher, when the fermentation is carried out by yeast low in nitrogen, low ferment power, working in the presence of a large amount of sugar (the proportion of 1 yeast for 5 of sugar is very favorable). On the other hand, when the fermentation takes place rapidly, via a large quantity of yeast rich in nitrogen, the decomposition of the amino acids is relatively weak (Ehrlich).
The proportion of the higher alcohols formed also depends on the composition of the must in nitrogenous matter. Yeast uses amino acids only if it does not have another source of nitrogen that is more easily assimilated.
When adding to the must, asparagine, or better, carbonate or sulphate of ammonia, the production of fusel-oil is very much diminished, even in the presence of large quantities of leucine (Ehrlich, Prings heim). However, it is not possible to completely suppress this production. Pringsheim (4) found that a dose of 250 gr. Ammonium sulphate per 1,000 liters of must was enough to reduce to a minimum the quantity of higher alcohols formed; by doubling the dose of ammonia salt, the rate of fusel remains the same.
(4) Biochem. Z. III, 121, 1907 : X, 490, 1908.
On the other hand, the addition of amino acids, and more particularly leucine, to a sugar solution increases the production of fusel oil, the proportion of which could, according to Ehrlich, be 3% under favorable conditions. The amount of higher alcohols, however, is still limited here, because the yeast uses amino acids only in so far as it needs their nitrogen to build the protein materials used in the constitution of its cells. The excess of amino acids remains unconverted.
Thus, Houssiau observed that the nitrogen of the amino acids disappearing during the fermentation (must of molasses, cane and beet) was 30 to 60%, and Martraire that the amount of amino acids used was sensibly constant. (0.050 gr of N per liter), whatever the initial content of the liquid in amino acids.
Under certain conditions, the production of higher alcohols could, however, be much greater. Salesskaja (5) obtained with musts containing 10% sugar, largely aerated and added with 0.5% leucine, a fusel-oil yield of 13.5% of the alcohol or 0.16% of the must, which represents an assimilation of 70 to 80% of the amino nitrogen. Increasing the amount of leucine does not cause the increase in the level of higher alcohols. The replacement of leucine by another amino acid lowers the proportion of fusel oil to 0.08 and even 0.04% of must. [I think I translated the logic of that correctly.]
(5) Mikrobiologija VI, 68, VII, 66, 1938.
Yeast does not use only the naturally occurring amino acids in musts, but also those derived from the disassimilation of the nitrogenous principles and the autolysis of old cells. Thus, some authors explain the formation of isobutyl alcohol from valine, a normal product of the degradation of yeast albumins. However, according to Ehrlich, the amino acids excreted by yeast would only be of great value if, as a result of defective nitrogen nutrition, high temperature or other adverse conditions, Partial autolysis of the old cells occurs.
The composition of the fusel-oil varies within wide limits, according to the nature of the amino acids of musts, the conditions under which fermentation takes place, etc. In general, there is dominance of isoamyl alcohol, which sometimes constitutes up to 80% of the fusel oil, with varying amounts of normal isobutyl and propyl alcohol. Butyl and isopropyl alcohols are the result of bacterial interventions; they can be found in large quantities in alcohols from must left for spontaneous fermentation.
Kayser and Demolon have observed that the production of higher alcohols is favored by light and that of volatile acids by darkness. According to these authors, the formation of higher alcohols would be related to the yeast multiplication intensity. It is, in fact, increased in the presence of high doses of peptone, which promotes cell multiplication and decreased if we add to the must of asparagine, which increases the speed of fermentation by reducing the yield of yeast. In other cases, it has been found that the amount of higher alcohols formed varied in the same direction as the weight of yeast recovered.
Jankovic (1) has observed that factors favoring the activity of yeast also increase the production of fusel oil. Having fermented a molasses must (which contained 52.4% sugars and 1.7% nitrogen) under different conditions of concentration and temperature, he found that the maximum amount of fusel oil (1.45%) was obtained when the density of the must was 15° Brix, the acidity 0.5 and the temperature 27°; and the minimum amount (0.22%), if the density was raised to 25° Brix and the acidity to 1.5, the temperature remaining at 27° C.
(1) Z. Spiritusind. LI, 106, 1928.
The different races of yeast seem to be rather little differentiated in the production of the fusel oil, according to Kayser and Demolon. However, Schizosaccharomyces gives lower amounts of higher alcohols than budding yeasts (Kayser).
On the other hand, Arroyo found notable differences in the production of higher alcohols, according to the yeast races. He obtained the following results, using cane juice as a culture medium and operating at a temperature of 33-35°.
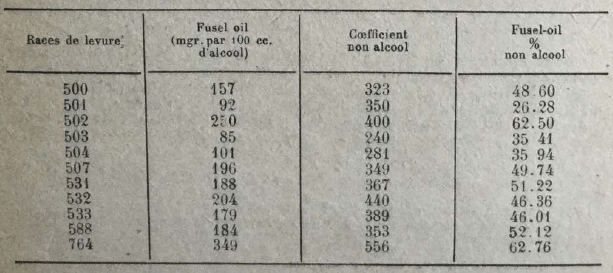
The same author has also observed that the formation of higher alcohols diminishes when the fermentation takes place at relatively low temperatures and when yeast is fed with liquid ammonia in place of the sulphate of ammonia. Hereinafter, the amounts of higher alcohols obtained in various tests (in grams per hl. alcohol at 100 °).

Methyl alcohol [Methanol]. As a result of the works of Fellenberg, Buchka, Takahashi, Flanzy, etc., we tend to admit that methyl alcohol is found in variable quantities, but generally low, in all liquids which have undergone alcoholic fermentation.
Von Fellenberg (1) showed that pectins could be considered as methyl esters of pectic acids and contained on average 10% of methyl alcohol. Under the influence of various causes (heating with dilute acids, alcoholic fermentation, etc.), the pectic substances are decomposed, with release of the methyl alcohol. Pectoses and lignins can also give birth to this alcohol, but more difficult than pectins.
(1) Mitt. Lebennsm. Hyg. V, 172, 1914.
According to Flanzy, during the alcoholic fermentation, and in the absence of any pectic product, a certain quantity of methyl alcohol, variable with the nature of the sugar, would be produced: glucose, for example, would give more methyl alcohol than levulose.
It would be especially at the beginning of the fermentation that methyl alcohol would appear, at the same time that the pectic compounds undergo a profound modification resulting in their de-methylation and the liberation of their constitutive phosphorus (Flanzy).
Acids. — The acidity of fermented liquids is due to fixed (lactic acid, succinic acid, etc.) or volatile acids (formic acid, acetic acid, butyric acid, etc.) brought by the raw materials of the must or produced during fermentation.
According to Arroyo, the quantity of acids formed by the yeast during fermentation is usually the equivalent of 4.7 to 10.0 cc of decinormal soda per 100 cc. of must. Volatile acids correspond to 2.1-5.8 and acids to 2.1-5.4 cc. decinormal soda. [this is the baseline that the Δ acidity is abased on.]
The presence of succinic acid, CO2H-H2-CH2-CO2H, among the products of alcoholic fermentation has been reported for the first time by Pasteur. The amount of this acid (usually 0.5 to 0.8% of processed sugar) is very variable. It depends mainly on the race of yeast and the primitive acidity of the must (Ventre).
Pasteur thought that succinic acid resulted from the processing of sugar. It is admitted today, following the works of Ehrlich and Neuberg, that it comes from the decomposition of the proteic matters, under the influence of the yeasts or the microbes of the putrefaction. It appears to derive mainly from glutamic acid, by a process analogous to that of the formation of higher alcohols from amino acids:
![]()
While succinic acid appears at the end of fermentation, volatile acids are formed during the early stages of fermentation (Reisch, Oster walder); their production ceases practically when the amount of sugar has been reduced to 50% (Joslyn and Dunn). However, when the fermentation is finished, they increase again, as a result of the oxidation of the alcohol. Osterwalder (1), confirming the results of Kayser and Demolon, showed that yeasts preserved in the fermented must could produce as much as 1.8 per 1,000 of volatile acids.
(1) Cent. Bakt. Abt. II, XXXII, 481.
The quantity and the nature of the volatile acids vary not only with the various factors which act on the development of the ferments (reaction of the medium, temperature, aeration), but again, especially with the yeast races.
Fernbach (2) has shown that the total acidity formed during the fermentation was all the greater when the liquid was originally less acidic: in the tests carried out, it varied from 59.5 to 123.5 mgr (tartaric acid) liter, when the original must was neutral.
(2) C. R. CLVI, 77, 113.
Ventre (3) observed in the case of neutral grape musts, a fixed acid production of 1-1.7% of the sugar consumed, each yeast having a particular way of forming fixed acidity and volatile acidity. In acidic environments, however, there may be a decrease in acidity during fermentation, as a result of the yeast attack of certain organic acids (primarily malic acids) in the wort. [This may be a paper worth seeking out to see if it refers to S. Pombe yeasts metabolizing malic acid.]
(3) C. R. CLVII, 154, 1913.
Kayser found that the quantity and also the nature of the volatile acids produced depended to a large extent on the yeast race. Yeasts á voile [you’d think airborn—sailing wild yeast, but likely it translates as veil like the film yeast of Vin á voile], for example, give rise to large quantities of acids, with notable production of formic acid. The nitrogen diet also has a significant influence, but varies according to the races in the ferment.
According to the same author, the main volatile acids which originate in the fermentation of cane molasses by pure yeasts are acetic, butyric and formic acids. The first of these acids is in general largely predominant and sometimes alone accounts for almost all of the volatile acidity. Formic acid, however, would be produced in appreciable quantity by certain yeasts (veil yeasts) and under certain conditions of nitrogen supply (nitrogen amide). According to Thomas (4), if yeast is cultivated in the presence of acetamide or time, formic acid is formed almost exclusively.
(4) Ann. Inst. Pasteur XXXIV, 162, 1920.
Srinivasan found in fermented cane molasses, besides formic, acetic and butyric acids, propionic acid in noticeable quantities; and various authors have reported the presence, in fermented liquids and eaux-de-vie, of high molecular weight fatty acids: caproic. capric, lauric, palmitic, etc.
It is now admitted that acetic acid is a side product of the conversion of sugar into alcohol and that it results from the oxidation of the acetic aldehyde (Kostyjschew, Neuberg). It can also come from the oxidation of ethyl alcohol by the yeast after fermentation (Kayser and Demolon, Osterwalder). However in practice, the presence of significant amounts of this acid is always related to the intervention of acetic bacteria, parallel or subsequent to the alcoholic fermentation.
According to Thomas, the formic acid results from the protoplasmic activity of the yeast, oriented in a particular direction by the supplied nitrogenous foods. It may also derive, at least in the case of molasses musts, directly from sugar by a purely chemical route (Wilson).
According to a widespread opinion. the other acids which are formed in the alcoholic fermentation would come from the dis-assimilation of the yeast cells. Their formation was attributed by Duclaux to the mechanism of nitrogen nutrition.
Thus, ketone acids from the conversion of amino acids, instead of reducing the higher alcohols, can, under certain conditions, oxidize acids having fewer carbon atoms than amino acid from which they come:
![]()
The hydrolysis of certain amides also sometimes gives rise directly to acids:
![]()
E. Luce (1) admits that the acids also come, at least partly, from the fats of the yeast. These would first be split into glycerin and fatty acids, which would then be degraded, as in Knop’s ß oxidation theory.
(1) J. Pharm, Chim, (7), XXII, 138, 1920.
Under the influence of oxidants, the normal acyclic acids are converted into oxacids, corresponding to the β-, that is to say on the penultimate-carbon, oxal forming OH group: [SOS not sure if this translated correctly, a tricky one.]
![]()
Under the action of a slightly energetic oxidation, the secondary alcohol group CHOH is converted into an acid group CO2H, the bicarbonated chain CH2 – CO2H becoming detached from the molecule, which decreases by 2 the number of atoms of carbon from it.
![]()
Depending on the conditions of the fermentation, the chain would be cut into more or less short sections.
Finally, the raw materials used in the composition of musts provide certain higher fatty acids (palmitic acid, oleic acid, etc.), which, being volatile with water vapor, can be found in the distillate.
Esters. — If, during the distillation and storage of alcoholic liquids, a certain quantity of esters is produced chemically, it is especially during fermentation that these compounds are produced by biological means.
Kayser and Demolon (1909) observed that the quantity of volatile esters formed during the fermentation was substantially proportional to that of the volatile acids produced by the yeast; that the addition of acetic acid, either before fermentation or immediately afterwards, in no way increased the proportion of the esters; and, finally, that, by distilling the yeast collected immediately after fermentation, a certain quantity of esters was always obtained, it was concluded that the esters were endogenous formation products.
This opinion, already issued by Ashby in 1907, was confirmed by the work of Ribereau-Gayon and Peynaud, Chai-Heung-Kim (2), and so on. It is currently accepted that the esterification, physiologically during the fermentation is carried out under the action of a special diastase, esterase.
(2) Enzymologia, VI, 3, 183, 1939.
The esters formed gradually diffuse into the liquids, especially at the end of fermentation, and can be destroyed over time by the yeast itself. Highly volatile products such as ethyl acetate disappear for a very high proportion, either by entrainment of carbon dioxide, or especially by evaporation in contact with air (3) (Kayser and Demolon).
(3) The vapor pressure of ethyl acetate at ordinary temperature is about twice that of alcohol, although the boiling points of these bodies are very close.
The nature and proportions of the esters produced depend chiefly, as Kayser has shown, on fermented races and bacteria. Nitrogen nutrition of yeast and acidity are also important.
The dominant ester is ethyl acetate, which often forms up to 98% of the total esters. The ethyl formate can thus exist in significant amounts, especially in molasses musts, as well as ethyl butyrate. Finally, there are small amounts of high molecular weight fatty acid esters (caproate, caprate, laurate, etc.).
Ashby isolated Jamaican molasses, a very slow fermenting yeast, giving per liter of alcohol at 100° to 18,000 mgr esters, consisting mainly of ethyl acetate. N. Deerr found in a molasses of Natal a race of Monilia providing, in pure culture, by liter of alcohol 7,550 mgr of esters, mainly formed by acetate or ethyl butyrate. [SOS not sure of this last line. Does he mean ethyl acetate AND ethyl butyrate?]
Kayser has also observed that some yeasts, are likely to provide very large quantities of esters. This author obtained the following results, by fermenting molasses must at 14% (by volume) with, on the one hand, a low yeast [bottom fermenting?] of Guadeloupe (A), and on the other hand the same yeast associated with a veil yeast [film yeast] from Réunion. The analysis was done after 15 days. [Percival Greg’s yeast #18 was a top fermenting Pombe yeast that formed a yellow head. It could be what they are describing. These guys were eye witnesses to a lot more variety.]

In another trial, by seeding the same molasses must with the combination of low yeast and veil yeast simultaneously (A); with the low yeast and, once fermentation in full activity, with the high yeast (B), the results were as follows (analysis made after 3 weeks): [I think the distinction here again is top fermenting and bottom fermenting.]

The following experiment by the same author shows the influence of nitrogen feed on the production of volatile acids and esters. The must of molasses, which contained per liter: 150 gr. of sucrose, 1 gr. of acid phosphate of K and traces of Mg sulphate, was added 0.5 gr. of nitrogen in the form of eau de touraillon [this may have been water from grains used as a nutrient], peptone, asparagine, leucine, urea, glycine and sulphate of ammonia. Yeast 3 was a veil yeast derived from a cane molasses from Reunion, and the yeast 5 another veil yeast isolated from the pineapple. The liquid was analyzed after 15 days of fermentation.

These results show that the nitrogen supply of yeast has a very clear, but also very variable, influence on the quantities of volatile acids and esters obtained. The proportions existing between the different acids and esters are also influenced (Kayser).
Lindner, studying 12 pressed yeasts, found that esters were formed mostly in well-aerated liquids, low in nitrogen, or when the growth of yeast was stimulated by a large excess of sugar or when fermentation took place at high temperature. The property of producing aromatic esters was manifested mainly in yeasts of secondary fermentation, living at the expense of old globules. [old globules may be old yeast cells?]
Finally, it should be pointed out that hydrogen sulphide resulting from the yeast reduction of sulphites or sulphates of the culture medium can, under certain conditions, give ethylsulfhydric or mercaptan ester with alcohol. The presence of the latter has been reported in some wines when fermentation took place in the presence of sulfur, sulphurous acid, or sulphate. The production of mercaptan depends on the race of yeast and the conditions of the medium: it increases with the temperature and the presence of easily reducible products. It appears to take place especially after the active phase of fermentation, when the yeast has a more energetic esterifying action.
The transformation of sugar according to the theoretical formula of Gay-Lussac would give, for various fermentable carbohydrates, the following yields in alcohol (liters of pure alcohol per 100 kgs of sugar):

However, as it always happens, beside the transformation of sugar into alcohol, the “fermentation derivative”, the importance of which varies with the operating conditions, the actual yield is always lower than the preceding figures.
Pasteur (1), after several careful dosages, had come to the conclusion that about 6% of the sugar was used for purposes other than the production of alcohol. Operating with 100 gr. of sucrose, dissolved in an appropriate mineral medium, he found as duplicating products (in gr.):
(1) Pasteur (L.) – Mémoire sur la fermentation, alcoolique. Ann. Chim. Phys. LVII et LVII. 1859.

The surplus of 5.65 gr. of the 100 gr. sugar introduced comes from the water fixation that accompanies the hydrolysis of sucrose. 100 gr. of sucrose thus giving 105.21 gr. of glucose, there remains a small difference of 0.39 gr., which may be due to the hydrolysis of some by-products during fermentation.
Although representing an experimental result, the Pasteur figure (64.33 liters of alcohol per 100 kgs of sucrose) has been considered by some authors as a “theoretical yield”, impossible to exceed and even to achieve under the conditions of industrial practice.
It has been found, however, that the rate of by-products of fermentation can be reduced under certain conditions. Effront (1) in particular showed that the use of yeasts accustomed to hydrofluoric acid made it possible to reduce the amount of glycerine produced to one tenth of that obtained using ordinary yeasts.
(1) Influence de l’acide fluorhydrique et des fluorures sur les levures de bière. C. R. CXVII, 183 — CXVIII, 1894.
On the other hand, an appreciable part of the sugar diverted from the alcoholic production is used for the construction of the yeast cells (cellulose and indeterminate matters of the balance of Pasteur). Duclaux (2), on the basis of Pasteur’s work, estimates the weight of sugar required for the formation of 1 gr to be between 1.5 and 2 gr. dry yeast. According to Claassen, this figure would be equal to 2 gr. However, it is possible to save the sugar used for cell reproduction. Brown and Pedersen have indeed shown that the amount of yeast used to inoculate a must has no influence on the final number of globules, which remains about the same per unit volume. This maximum number of yeasts has been called “Brown’s Limit Number”, or “Specific Cell Saturation” (Boinot). If a quantity of yeast seed containing a number of cells close to the limit number is introduced into a must, little or no new cells are produced, and the construction costs are consequently very low or nil.
(2) Traité de Microbiologie : S t. III. La fermentation alcoolique, Paris 1905.
It was objected that the total expenditure on the maintenance of yeast cells was therefore greatly increased (Bettinger). The number of globules per liter of must is usually, in fact, in the usual methods of fermentation, 150 billion, which corresponds to 3.1 gr. dry yeast. Under certain conditions, especially when using antiseptics, the figure can be lowered to 100 and even 80 billion (2 gr and 1.67 gr of dry yeast). Now, if the fermentation is made by seeding the must with a quantity of cells corresponding to the Brown limit, the number of globules is increased to 550 billion (11.46 grammes of dry yeast per liter), according to the observations of Grimaud.
This objection has been answered that the additional amount of sugar used in this case for unproductive maintenance expenditure is, however, much less important than that used in other processes for cell multiplication (3). In any case, Boinot (4), by fermenting in the laboratory various samples of beetroot molasses from France and cane molasses from Cuba and South Africa, by recovery of yeasts, was able to obtain yields varying between 64.5 l. and 66.9 l. alcohol per 100 kgs of vanished sugars (expressed as sucrose), all exceeding the Pasteur coefficient.
(3) By the method of recovery of yeasts, the duration of the fermentation is, in fact, reduced by about 40%. In addition, it appears that the unitary maintenance expenditure is even higher as the number of cells decreases (Pérard).
(4) Bull. Ass. Chim. LVI, 728, 1939.
To the above causes of loss are added, in industrial fermentations, those due to the intervention of foreign ferments (wild yeasts, mycoderms, bacteria), which attack the sugar but giving little or no alcohol: conditions (temperature, acidity, composition of musts, etc.) adversely influencing the alcoholic function of the yeast; the presence in the must of non-fermentable sugars up to 3-4% of total sugars); to evaporation and entrainment by carbon dioxide (1% or more of the alcohol produced), when fermentations are made in open tanks. Also, the yields generally remain very much lower than the Pasteur coefficient. In the case of cane molasses processed by the various fermentation processes, the following percentages of the Pasteur coefficient would be obtained according to Magne:

1 thought on “Kervegant P. 51-80”